Two emerging therapies received the Food and Drug Administration’s (FDA) “Breakthrough” designation for the treatment of food allergy this year. We’ll take a look at what it means to be a breakthrough therapy, who is developing these them, how they work, and the (big) business drivers behind them.
The Food and Drug Administration Safety and Innovation Act (FDASIA) was signed into law in July 9, 2012. Section 902 of the legislation provides for a new fast track designation – Breakthrough Therapy. According to the FDA, a breakthrough therapy is a drug:
- intended for use alone or in combination with one or more other drugs to treat a serious or life threatening disease or condition and
- preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development.
A drug given such a designation is provided expedited review by the FDA, though the sponsor must still demonstrate that it is effective and safe. The FDA assigns senior resources to work with the sponsor on a continual basis to speed up the entire process from clinical trials through approval.
Both therapies are suppressive immunotherapies that work in a similar manner but are delivered via different mechanisms; they train the immune system to suppress immune system responses that cause allergic reactions by introducing an allergen or antigen into the body starting with tiny doses that increase over time.
This April, DBV Technologies – a publicly traded firm headquartered in Bagneux, France – announced they had received breakthrough designation for their Viaskin® Peanut therapy.
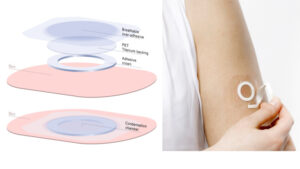 The delivery mechanism, dubbed epicutaneous immunotherapy (EPIT®) introduces the active compounds via a patch worn on the skin, similar to a nicotine patch worn by ex-smokers. Over time, successive patches deliver increasing quantities of the compound through the intact skin to be absorbed by the body before it reaches the bloodstream and causes a severe reaction.
The delivery mechanism, dubbed epicutaneous immunotherapy (EPIT®) introduces the active compounds via a patch worn on the skin, similar to a nicotine patch worn by ex-smokers. Over time, successive patches deliver increasing quantities of the compound through the intact skin to be absorbed by the body before it reaches the bloodstream and causes a severe reaction.
The Viaskin® Peanut therapy is scheduled begin Phase III trials – the final step during which it will be tested on a larger group to determine its efficacy and safety – this quarter. If all goes according to plan, DBV Technologies hopes to begin marketing the product in the US sometime in the first half of 2018. The firm has similar therapies for cow’s milk and egg in earlier trial phases.
This June, Aimmune Therapeutics – a privately held biopharmaceutical firm headquartered in Brisbane, CA – announced they had received breakthrough designation for AR101, their Oral Immunotherapy (OIT) peanut therapy.
 The more familiar delivery mechanism introduces a standardized peanut flour that is mixed with food and ingested by the patient, with the dose of peanut protein increased over time.
The more familiar delivery mechanism introduces a standardized peanut flour that is mixed with food and ingested by the patient, with the dose of peanut protein increased over time.
The AR101 therapy is scheduled to begin Phase III trials this quarter as well. Aimmune expects to begin trials of therapies addressing other allergens in the future, though they have not yet disclosed which.
What is the incentive for these companies and others to develop therapies? In a recent Reuters article, analysts estimated that a year’s supply of Viaskin will cost $6,500 and Aimmune’s treatment will cost $5,500. The article quotes Credit Suisse analyst Vamil Diwan estimating that AR101 could could reach peak annual US sales of $1.33 billion, while analysts at Morgan Stanley and Jefferies estimate Viaskin could reach potential for annual sales of greater than $2 billion.
While we are hopeful that these therapies and others will prove safe and effective and delivered within the expected timeframes, we also hope health insurers will move quickly toward approving these therapies to help defray the cost to families coping with food allergy, and that by that time all families will be covered by health insurance.
- Fact Sheet: Breakthrough Therapies – FDA
- Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics – FDA
- DBV Technologies Receives FDA Breakthrough Therapy Designation for Viaskin Peanut for the Treatment of Peanut Allergy in Children – GlobeNewswire
- Peanut allergies are a serious and growing health problem in the Western world, affecting millions of kids and their families – CNN Money
- Aimmune Therapeutics Receives FDA Breakthrough Therapy Designation for AR101 – Yahoo Finance
- Viaskin Products Pipeline – DBV Technologies
- Developing a standardized, pharmaceutical-grade peanut protein formulation for the treatment of peanut allergy – Aimmune Therapeutics
- Biotech companies chase elusive peanut allergy treatment – Reuters





Thanks for the informative article! There are a few more points to add about Aimmune:
Our Planters peanuts cost $4/month or $50 a year for 10 peanut-level protection. Be VERY careful with the FDA peanut capsule by Aimmune. Allergist are in for a real shock with the FDA version! OIT is just a protocol with food–the FDA doesn’t approve protocols. So they had to re-engineer regular old peanut flour to get a patent on the product. They also picked a random protocol rather than testing all the different ones out there. So here are some key points:
First, it starts at a high dosage of .5mg versus most protocols that start much lower like .02mg because they are liquid dilutions not flour.
Second, be careful because the Day 1 final dose is 6mg where most now stop at 3mg max. So kids will get sick in the first week at such a high dose by starting at 6 mg.
Third, it only takes you to 1 peanut’s worth of flour. That’s not much protection so you have to maintain STRICT AVOIDANCE forever, unlike real OIT where the result is total freedom from food fear and even being able to eat it if you want.
And lastly, the biggest shocker of the fake peanut pill is the MISSING PROTEINS. Yes, they REMOVED them so they could patent the peanut flour as a biological drug. Scary and of course dangerous. “Second, the peanut-in-a-pill only contains three of the four storage proteins associated with anaphylaxis, Arah 1, 2, and 6, but not 3, and neither the birch pollen analogue Arah 8 nor the lipid transfer protein Arah 9, which are in the whole package you get from Planters. This was done because otherwise one of the world’s most ubiquitous foodstuffs wouldn’t be patentable. The three-component combo is now locked up, like snap, crackle, and pop. If it works as described, many patients will be “bite proof” (able to tolerate a trace exposure) but not all. Maybe the firm intends to create another pill with the missing components, as they prepare to roll out one allergen after another.”
“A further concern is what will happen to patients after they have reached maintenance. Will they have to continue taking only their three-component designer drug, will they be able to shift to generic peanuts, or will they be able to eat Thai food a few times a week? What will happen when they exceed the bite-proof threshold achieved with ARA101? Will they have anaphylaxis? Will their mouths itch because the drug has done nothing for the components other than 1, 2, and 6?”
Luckily, a good allergist can just use real peanut flour and a protocol and get safer,cheaper and better results for you.
Full article: http://asthmaallergieschildren.com/2015/09/10/new-peanut-therapy-gets-fast-tracked/#sthash.sGHDH1II.dpuf