Home Search
viaskin - search results
If you're not happy with the results, please do another search
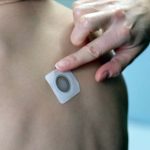
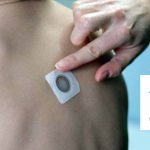
Viaskin Peanut “Patch” Therapy Takes Next Step Toward Approval… in Europe
The Marketing Authorization Application for Viaskin™ Peanut was validated by the European Medicines Agency.
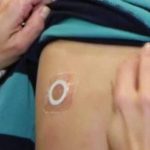
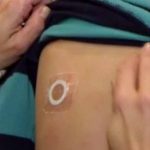
FDA Denies Approval of Viaskin Peanut “Patch” Therapy
The FDA raised concerns that adhesion of the patch would affect the efficacy of the therapy.
Will the Viaskin “Patch” Therapy for Peanut Allergy be Approved Soon? The Company is...
Company is restructuring in anticipation of delays.
FDA Has Questions About Viaskin Peanut Efficacy, Delays Meeting with DBV Technologies
The company warned the FDA's target date for their decision on approval could be delayed.
FDA Advisory Committee Meeting to Review Viaskin Peanut for the Treatment of Peanut Allergy...
Next step in the approval process for the therapy scheduled for May 15, 2020.
DBV Technologies Reports Positive Three-Year, Long Term Data from Phase III Open-Label Extension Study...
Patients demonstrated durable, long-term clinical benefit with an additional two years of treatment.
DBV Technologies Announces FDA Acceptance of BLA filing for Viaskin Peanut for the Treatment...
If approved, Viaskin Peanut would be the first and only epicutaneous immunotherapy indicated for this potentially life-threatening condition in children.
DBV Technologies Submits Biologics License Application to FDA for Viaskin Peanut for the Treatment...
This submission addresses the additional data needed on manufacturing procedures and quality controls which were communicated by the FDA in December.
DBV to Resubmit Application to FDA for Viaskin Peanut Approval in Q3 2019
Company allays concerns that BLA would not be resubmitted.
DBV Withdraws FDA Application for Viaskin Peanut with Plan to Resubmit
The company believes the additional information needed to support this filing is available without further clinical studies.