Since the FDA approved neffy™ on Friday, we’ve been inundated with questions from our readers.
To get definitive answers, we reached out to ARS Pharmaceuticals — the company that developed neffy — to give us the scoop.
What is neffy?
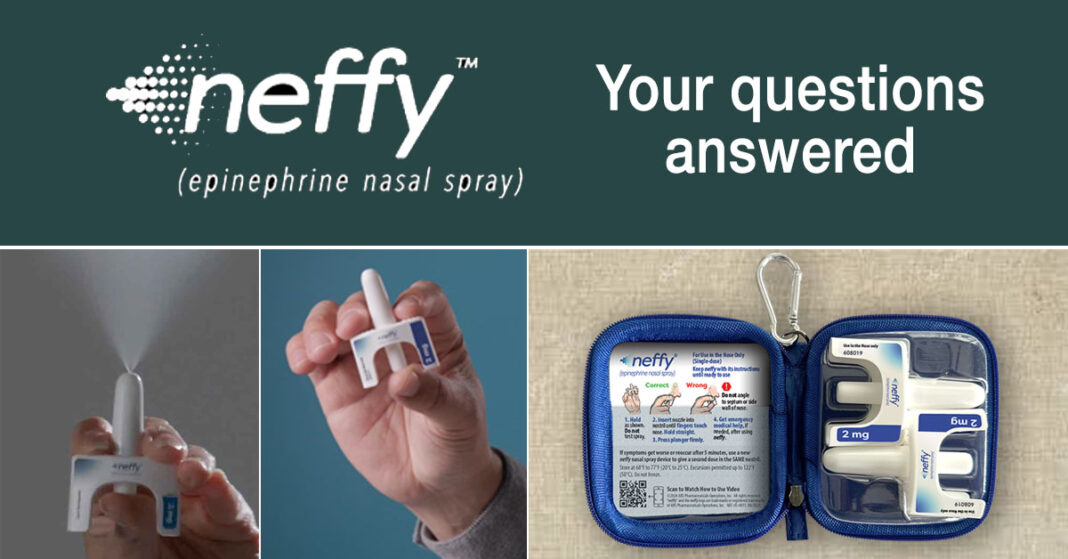
Neffy is an emergency epinephrine device used to halt and reverse the progression of anaphylaxis. Rather than deliver epinephrine via a jab to the outer thigh like traditional auto-injectors, neffy delivers a fine mist sprayed into a nostril. As such, it is the first FDA-approved first-line needle-free emergency epinephrine device.
Is neffy as effective as an auto-injector?
Yes, neffy was designed to deliver epinephrine within the range of injectable products and has demonstrated rapid onset and short time to resolution of symptoms in patients with anaphylaxis.
Is neffy effective if the patient has nasal congestion?
Neffy is meant to coat the nasal membranes, not to be inhaled into the lungs. Because nasal membranes are often inflamed with congestion, the drug was actually found to be absorbed better with congestion.
Can neffy be administered if the patient is unresponsive?
Yes.
When can I begin filling prescriptions for neffy?
The company hopes to have drug available through their specialty pharmacy and retail pharmacies before the end of September.
Patients and caregivers can register now on www.neffy.com via neffyConnect early this week.
What is neffyConnect?
NeffyConnect is a specialty direct-order pharmacy that will fill prescriptions in one of three ways:
- By receiving a prescription from the patient’s doctor;
- By requesting a prescription from the patient’s doctor;
- Via a virtual physician that can prescribe neffy for you [available within 3 weeks].
neffyConnect will ship the device to the patient/caregiver as soon as it becomes available, and is currently the fastest way to receive it.
Will the physician need to prescribe neffy specifically, or will it be fillable with an auto-injector prescription?
Neffy must be specifically prescribed; it cannot be filled with a prescription for a specific auto-injector. That said, patients will be able to access a virtual doctor via neffyConnect who can prescribe Neffy.
What is the time-to-expiration of the device?
The device is manufactured with a 30-month time-to-expiration but due to release testing and distribution, patients will receive neffy with an average of 24 months remaining, twice that of traditional auto-injectors.
What will neffy cost?
The company anticipates most people with insurance will pay $25 for a two-pack. Those without insurance or with high-deductible plans will never pay more than $199. Although this is roughly the same cost as that of generic auto-injectors, keep in mind that neffy will last twice as long if not used.
It may take some time for neffy to be added to the patient’s insurance company’s formulary requiring prior approval for the cost to be covered by insurance with a co-pay. If ordered via neffyConnect, ARS will do the work for the physician to obtain this prior approval.
What are acceptable temperatures for storage and excursions?
As with all medications, Neffy should be stored at room temperature. However, the labeling allows for excursions up to 122°F (50°C). So if neffy is accidentally left in a hot car for the day it should be fine.
While neffy states “Do not Freeze,” if accidentally frozen — which occurs below 5° F — it will not damage the product or device, but will take about 20 minutes to thaw. The device will not work until entirely thawed. Unlike an auto-injector, freezing does not damage the medication or the device.
How is neffy packaged?
Neffy is blister-packaged in a two-pack. Patients will be able to request a protective case from ARS. [See image above on lower right.]
When is FDA approval of neffy for children less than 30kg anticipated?
ARS anticipates filing for a 1mg dose for children 15-30kg within a month followed by the FDA taking up to 6 months to approve. The European Medical Agency and ARS are working toward a dosage for children less than 15kg which could take 2-3 years.