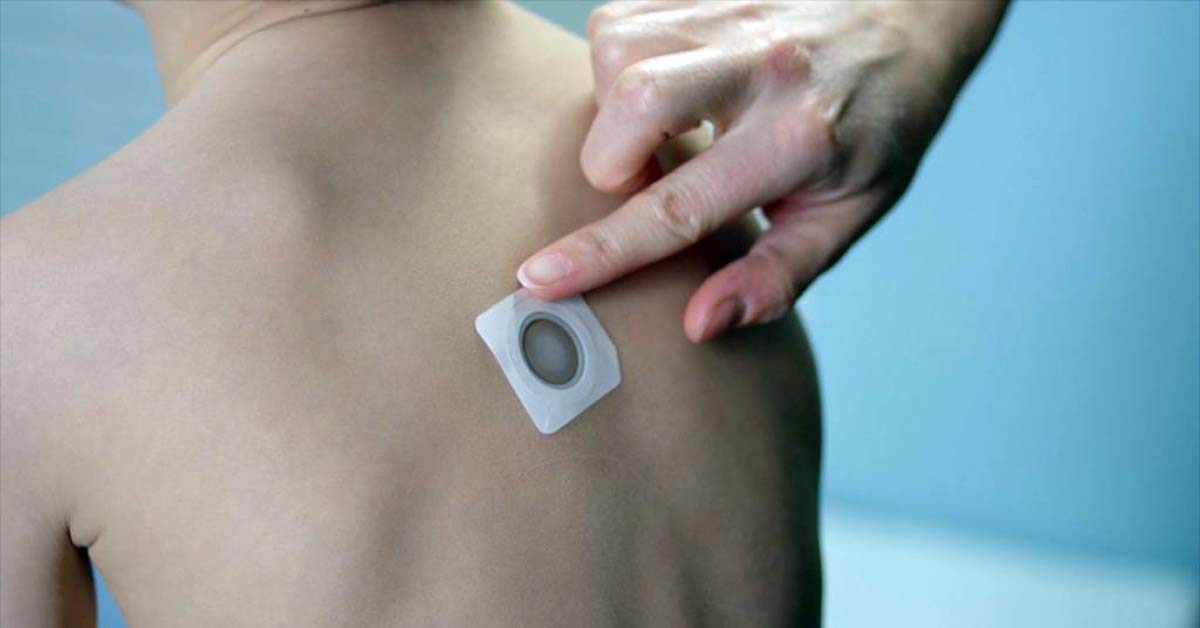
Viaskin Peanut is a novel immunotherapy applied to the skin via an adhesive patch. It is targeted at children 4-11 years of age who suffer from peanut allergy with similar Viaskin therapies in the pipeline to treat milk and egg allergies.
Recently the Food and Drug Administration (FDA) raised concerns that adhesion of the patch would affect the efficacy of the therapy, indicating the need for modifications and a new human factor study. The FDA decision will delay the US introduction of Viaskin Peanut for an indeterminate period of time.
That said, the therapy is moving ahead in Europe where it cleared the next hurdle, approval of their Marketing Authorization Application by the European Medicines Agency.
Here is the press release from DBV Technologies, the developer of the Viaskin family of treatments.
DBV Technologies Announces Filing and Validation of Marketing Authorization Application for Viaskin™ Peanut by European Medicines Agency
Montrouge, France, November 2, 2020
DBV Technologies (Euronext: DBV – ISIN: FR0010417345 – Nasdaq Stock Market: DBVT), a clinical-stage biopharmaceutical company, today announced that its Marketing Authorization Application (MAA) for its investigational product Viaskin™ Peanut (DBV712) has been validated by the European Medicines Agency (EMA). The validation of the MAA confirms that the submission is sufficiently complete to begin the formal review process for the investigational non-invasive, once-daily epicutaneous patch to treat peanut allergies in children ages 4 to 11 years.
Following the MAA validation, the EMA’s Committee for Medicinal Products for Human Use (CHMP) will review the application and provide a recommendation to the European Commission (EC) on whether to grant a marketing authorization. DBV expects to receive the first set of questions from the EMA approximately 120 days post-validation.
In August 2020, the Company announced that it had received a Complete Response Letter (CRL) from the U.S Food and Drug Administration (FDA) for its Biologics License Application (BLA) for investigational Viaskin™ Peanut. The Company is in the process of engaging FDA to discuss the regulatory path forward.
About DBV Technologies
DBV Technologies is developing Viaskin™, an investigational proprietary technology platform with broad potential applications in immunotherapy. Viaskin™ is based on epicutaneous immunotherapy, or EPIT™, DBV’s method of delivering biologically active compounds to the immune system through intact skin. With this new class of non-invasive product candidates, the Company is dedicated to safely transforming the care of food allergic patients. DBV’s food allergies programs include ongoing clinical trials of Viaskin™ Peanut. DBV Technologies has global headquarters in Montrouge, France and offices in Bagneux, France, and North American operations in Summit, NJ and New York, NY. The Company’s ordinary shares are traded on segment B of Euronext Paris (Ticker: DBV, ISIN code: FR0010417345) and the Company’s ADSs (each representing one-half of one ordinary share) are traded on the Nasdaq Global Select Market (Ticker: DBVT).
Forward Looking Statements
This press release may contain forward-looking statements and estimates, including statements regarding the therapeutic potential of Viaskin™ Peanut as a treatment for peanut-allergic children; anticipated regulatory interactions and procedures, including the expected timing of receipt of initial questions from EMA and the Company’s planned interactions with the FDA regarding investigational Viaskin™ Peanut; the implementation and impact of cost-reduction measures to be undertaken by the Company; and the advancement of the Company’s clinical development and regulatory review of investigational Viaskin™ Peanut. These forward-looking statements and estimates are not promises or guarantees and involve substantial risks and uncertainties. At this stage, the products of the Company have not been authorized for sale in any country. Among the factors that could cause actual results to differ materially from those described or projected herein include uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals, including the impact of the COVID-19 pandemic, and the Company’s ability to successfully execute on its restructuring plans. Furthermore, the timing of any action by any regulatory entity and possible regulatory paths forward with FDA cannot be guaranteed, particularly in light of the COVID-19 pandemic. A further list and description of these risks, uncertainties and other risks can be found in the Company’s regulatory filings with the French Autorité des Marchés Financiers, the Company’s Securities and Exchange Commission filings and reports, including in the Company’s Annual Report on Form 20-F for the year ended December 31, 2019, and future filings and reports by the Company. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements and estimates, which speak only as of the date hereof. Other than as required by applicable law, DBV Technologies undertakes no obligation to update or revise the information contained in this Press Release.
Investor Relations Contact
Anne Pollak
+ 1 (857) 529-2363
anne.pollak@dbv-technologies.com
Media Contact
Angela Marcucci
+ 1 (646) 842-2393
angela.marcucci@dbv-technologies.com