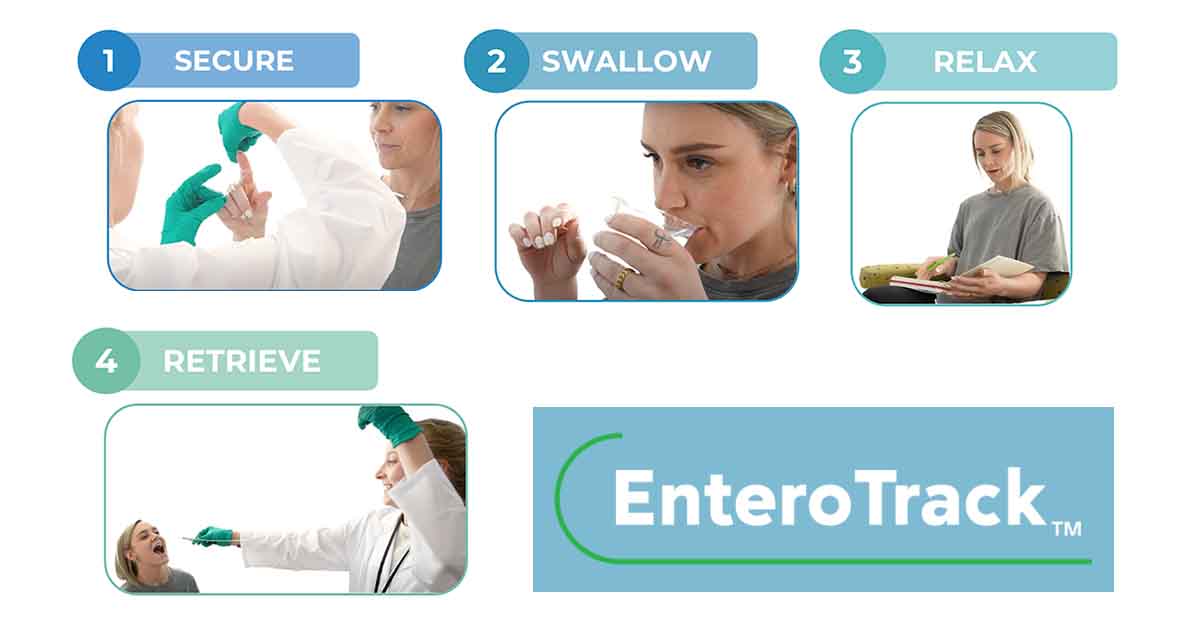
Test offers EoE patients a simple, clinically proven method to control their disease.
“…Beyond thrilled to use this at Phoenix Children’s Hospital…” — Dr. Schroeder, Medical Director, Eosinophilic Disease Clinic, Phoenix Children’s Hospital
AURORA, Colo., Oct. 24, 2022 (GLOBE NEWSWIRE) — Clinicians with the Gastrointestinal Eosinophilic Disease Program at the Digestive Health Institute, Children’s Hospital of Colorado, performed the Esophageal String Test® (EST) on a patient in early September. The test will be offered regularly at the hospital.
The EST was also performed on patients in the Eosinophilic Gastrointestinal Disease Clinic at Phoenix Children’s Hospital.
“I am now beyond thrilled to use this new esophageal surveillance technique at Phoenix Children’s and for my patients with eosinophilic gastrointestinal disorders. The EST is a game-changer for my patients who are looking for a less invasive and less expensive way to screen their disease activity,” said Dr. Shauna Schroeder, Medical Director for the Eosinophilic Gastrointestinal Disease Clinic at Phoenix Children’s Hospital. She further stated “Having trained in Denver, I had experience using the EST as a research tool in patients with eosinophilic esophagitis. I am now able to more granularly adjust medications and diets improving the care of my patients with eosinophilic esophagitis.”
The Esophageal String Test® utilizes a simple collection device, the EnteroTracker®, to collect upper gastrointestinal samples from adults and children. The samples are sent to an independent reference lab for analysis of disease activity. Several other clinics around the country are planning to offer the test soon.
About EnteroTrack
EnteroTrack, an early-stage company, develops simple-to-use, minimally invasive technologies to sample gastrointestinal (GI) mucosal content that can be assayed for various biomarkers of disease. The company’s platform technology, the EnteroTracker® is initially being used to support clinical monitoring of Eosinophilic Esophagitis in adults and children without need for sedation, advanced training, or complex procedures. Clinical studies evaluating the utility of the EnteroTracker® for additional applications including Esophageal Adenocarcinoma, Barrett’s Esophagus, GERD, GI microbiome, food allergy testing, and others are currently underway.
Contact Information:
Robin Shandas
CEO, Media and Investor Inquiries
robin.shandas@enterotrack.com
Brookelynn Stillwell
General Inquiries
brookelynn.stillwell@enterotrack.com