According to Food Allergy Research and Education (FARE), about 6.1 million Americans of all ages are allergic to milk. Most children, up to 75%, eventually outgrow their milk allergy, although the allergy is most likely to continue in children who have high levels of cow’s milk antibodies in their blood.
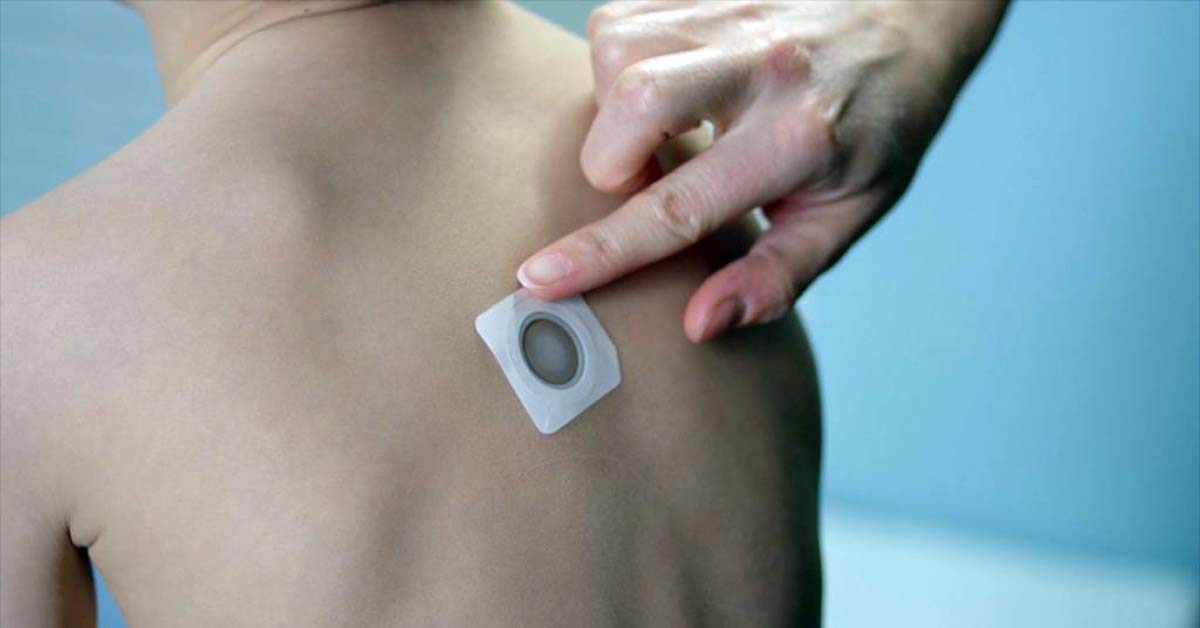
DBV Technologies, a biopharma company based in Bagneux, France, is developing a range of treatments for specific food allergies based on their Viaskin platform, delivered via a patch containing small quantities of allergens worn on the skin. Viaskin Peanut is in phase 3 clinical trials for children ages 1-11 years, and Viaskin Egg is in preclinical trials.
The company recently provided phase 2 clinical trial data for Viaskin Milk for children aged 2-17 years in the journal JAMA Pediatrics.
In this study, the safety and efficacy of the therapy were determined by comparing doses of 150µg, 300µg, and 500µg to placebo in 198 children with confirmed IgE-mediated allergy to milk. The primary outcome measured was the cumulative reactive dose of milk allergen determined with a 12-month food challenge.
95.5% of the participants completed the treatment, with most adverse effects being mild or moderate reactions at the application site.
The highest treatment response rate was 49% in children who received the 300µg dose compared to 30.2% in the placebo group. That response was higher for children aged 2-11 years who had a response rate of 57.9% at 300µg vs 32.5% receiving placebo.
Participants with severe life-threatening anaphylaxis to milk or uncontrolled persistent asthma were not eligible for participation, which could have affected the results and may limit generalizability, although these exclusions are common in immunotherapy trials for ethical concerns.
The number of adolescents in each treatment group was small, making it difficult to draw conclusions related to efficacy in this group. Moreover, patch placement was the inner side of the arms for adolescents, rather than the interscapular area, which may have also altered the response to treatment.
The researchers concluded:
In this dose-ranging randomized clinical trial of patients with [milk allergy], Viaskin milk at a dose of 300 μg resulted in statistically significant treatment response vs placebo following 12 months of therapy. These findings warrant further clinical trials to explore Viaskin milk as a viable treatment option for children with IgE-mediated [milk allergy].