I was contacted a number of months ago by Tal Golan, the CEO of an Israeli company called “Anjo”, who was soliciting feedback on a novel device they were developing. During a call, I was immediately struck by the breadth of the problem they were working to solve.
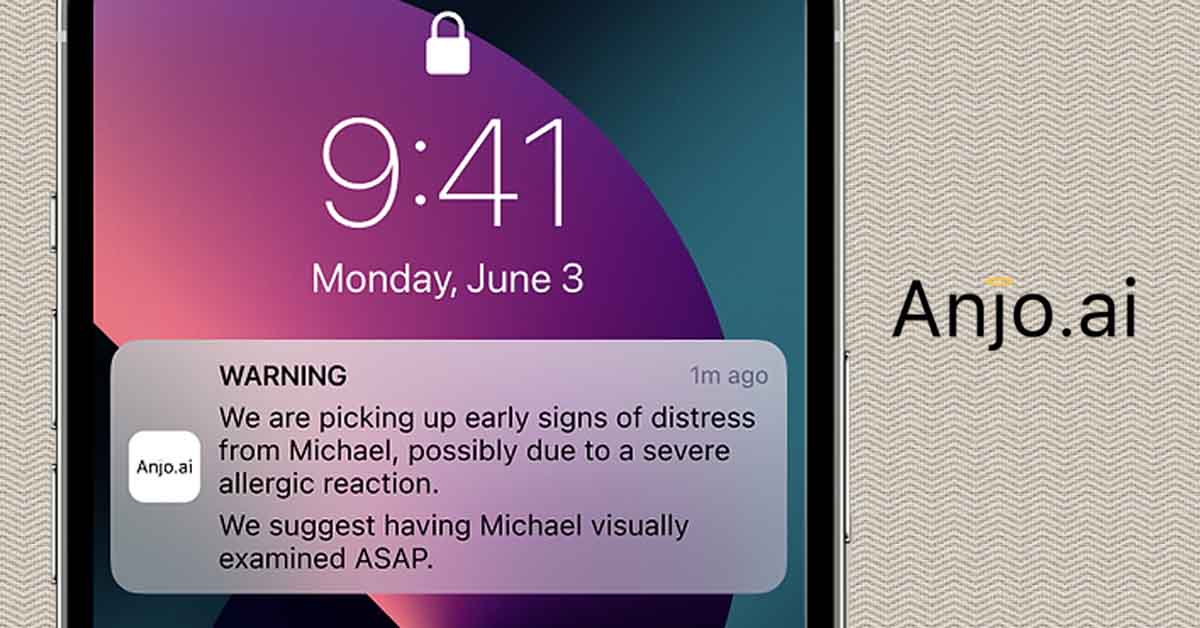
The company is developing a wearable device for children that will detect the symptoms of anaphylaxis and warn caregivers, possibly before the child even begins to show symptoms. If such a device were to come to market, it would be a boon to families of young children with food allergies everywhere.
Here is my interview with the principals of Anjo, who discuss their efforts and outlook.
Dave from SnackSafely.com: Welcome! Please introduce yourselves.
Itamar Nocham: Hello and thank you for this interview, Dave. Briefly about myself: I am a father to 3 children, currently living in Israel and my daughter has a severe milk allergy.
I have been a Systems Development lead and customer success professional for the past 20 years. I have worked with my co-founder, Tal, on various aviation safety systems projects. We both graduated from the same class in the Israeli Air Force Academy. I am the Chief Operating and Chief Product Officer for Anjo.ai.
Matan Lior: I am a father of 2 girls, currently living in Israel, and have been a software development and R&D team lead for the past 20 years, going all the way back to my military service. Tal and I grew up together and when Tal and Itamar approached me for this project, I was thrilled to bring my expertise to help families dealing with life-threatening allergies. I am Anjo’s Chief Technology Officer.
Tal Golan: I currently live in NJ, am a father to 2 boys, and have been a pilot for the past 22 years. Throughout my aviation career, I’ve also been involved in various Business Development roles, specifically advanced technologies such as future cockpits, safety systems, and sensors. I am the Chief Executive Officer at Anjo, and am thrilled and privileged to be working on such an impactful project alongside two extremely talented friends of mine.

Broadly speaking – what is Anjo? What makes it unique?
Tal: Anjo, which means angel in Portuguese, is the first unintrusive device for remote monitoring and early detection of anaphylaxis. It is the result of a combination of a proprietary algorithm, artificial intelligence, and data from clinical trials. This service will be available to all, no prescription required.
With Anjo, parents, teachers, school nurses, or any supervising adult that needs to know, is alerted when an allergic child begins showing early signs of distress, even when she/he is away from them. That way they can attend to them or make sure someone attends to them before it’s too late. I am sure readers here know how crucial it is to treat anaphylaxis as early as possible.
Our service will also include many allergy life-management features such as automatic logging of events, Epinephrine injector (or any future treatment) tracking, food labeling, etc.
The service will also include wellness and general health features such as activity, sleep, and stress tracking. All-in-one service, one app.
What problems facing the food allergy community do you hope to address?
Tal: Primarily, we hope to address the delayed treatment of anaphylaxis, which significantly increases the chances of prolonged hospitalization and even death. Also as a result of mitigating that risk, we hope to lessen parents’ anxiety when their allergic children are away from them.
We know of many cases where a teacher or parent missed the early signs, were unsure or hesitated, waited too long, or missed the critical time for treatment and unintentionally turned it into a life-threatening event. Sadly, some cases even resulted in a death that could have been prevented.
As noted, we’re also looking to address the stress and fear that parents experience when they send their allergic children to school, on playdates, or any activity away from them. The service we’re developing lets parents know at any time if their allergic children are well, in distress, or showing early signs of an anaphylactic event, even when they’re away from them.
It’s tough enough to deal with severe allergies as an adult, let alone when you need to protect and worry about your young child.
How did you come to decide to embark on the Anjo project?
Itamar: During my wife’s post-doctoral studies at Northwestern University, my daughter experienced an anaphylactic reaction at daycare. The school was very much aware of my daughter’s food allergy but somehow the teacher missed her early signs of distress. In fact, they were so late to notify us, we ended up rushing her to the ER where it took 3 doctors and multiple nurses a number of hours to get the reaction under control. It was so severe that the lead doctor told us he wasn’t sure she would make it. These were the toughest hours of our lives.
Tal: So Itamar and I decided to do something about it. We thought it was absurd that in this day and age, with food allergies being so pervasive, there is no way to know ahead of time and alert when a child is beginning to show signs of distress. We thought how absurd it is that parents still have to live in fear every time they send their allergic child to school, worry when the next anaphylaxis event will strike, and worry if the school staff will treat it on time.
When did you start working on Anjo in earnest and where are you now in the process?
Tal: We started Anjo earlier in 2022. We have a patent pending, two existing prototypes, and the foundation software for the upcoming medical studies and FDA clinical trials.
We’re launching our first study with the largest OFC and OIT clinic at the Shamir Medical Center in Israel in the next few weeks, and we recently received a request to partner with a large university here in the US as well. More relationships are in the pipeline.
What hurdles have you had to jump so far?
Matan: I have technological hurdles to jump every day! But we meet them as they arise and are making great progress.
What feature set do you have planned for the initial launch and how do you expect Anjo to evolve over the first few years?
Tal: For the initial launch, defined as Anjo 1.0, we are planning a Wellbeing and Abnormality notification service, meaning that the service will remotely alert the parent or caregiver of a substantial change in their child’s specific biomarkers, indicating that the child is in distress. Anjo 1.0 will be nonspecific to allergies, but will be the preface for Anjo 2.0, the fully developed FDA-Cleared Anaphylaxis Alert service. We’ve received feedback from schools that a live dashboard and abnormality notification for the allergic (or any high-risk) children at the school would be of great help. But we would also love to hear from readers here if they would like to have something of that sort for personal use as well.
Also, while simultaneously developing the core feature of an anaphylaxis alert, the initial service launch will include many life-management features that assist with everyday life for the allergic community.
What is left to do before Anjo is ready for prime time?
Tal: The list is long, but we are aiming to launch Anjo 1.0 in late 2023, then Anjo 2.0 later in 2024.
How will you test Anjo to build trust with the food allergy community?
Itamar: We are validating our technology at Oral Food Challenge clinics, and prior to releasing Anjo 2.0 we will undergo all FDA-required clinical trials.
Tell us about your team.
Tal: In addition to the founding team, we are fortunate to recently appoint Dr. Shira Goldman as our Chief Medical Officer. Dr. Goldman is an ER physician and a Pediatric Endocrinologist, she brings vast experience and perspective on the treatment and early identification of chronic diseases and life-threatening events. Aside from that, we will be expanding our team very soon both here in the US and in Israel.
Tell us what you can about fundraising. Is there an opportunity for our readers to invest in what you’re doing?
Tal: We are in the advanced stages of discussion with multiple investors and are currently evaluating our next steps. We’re always open to considering new opportunities, especially if they come from our food allergy community.
Is there a way for our readers to be involved in providing feedback?
Tal: Yes, please! We have prepared a brief questionnaire and welcome any feedback. For those who are kind enough to lend us five minutes of their time, we will be happy to add them to the Beta users list for free!
What would you like our readers to take away from this interview?
Itamar: We would like to remind everyone to carry their Epinephrine injectors with them and treat anaphylaxis as early as possible. Don’t wait until it’s too late!
Tal: Also, as readers may see here on SnackSafely, there are multiple organizations such as FARE, FASI, and others that are working diligently to help, educate and even solve the underlying causes of food allergies. And now with Anjo – more help is on the way!
Gentlemen, thanks for your time. Stop back in with us and let us know how Anjo is progressing! I’m looking forward to covering the completion of your milestones!
To learn more about Anjo, visit their website: anjo.ai. You can participate in their survey and join the list to become a beta tester by clicking here.