Moonlight Therapeutics, Inc., a biotechnology company developing targeted treatments for food allergies, announced today it successfully completed a pre-Investigational New Drug Application (pre-IND) meeting with the U.S. Food and Drug Administration (FDA) to discuss the regulatory pathway for the development of MOON101 for the treatment of peanut allergy. More specifically, Moonlight has received guidance from the FDA on a first-in-human clinical trial for MOON101 in peanut-allergic children and adults.
Current Federal law requires that a drug be the subject of an approved marketing application before it is transported or distributed across state lines. Because a sponsor will probably want to ship the investigational drug to clinical investigators in many states, it must seek an exemption from that legal requirement. The IND is the means through which the sponsor technically obtains this exemption from the FDA.
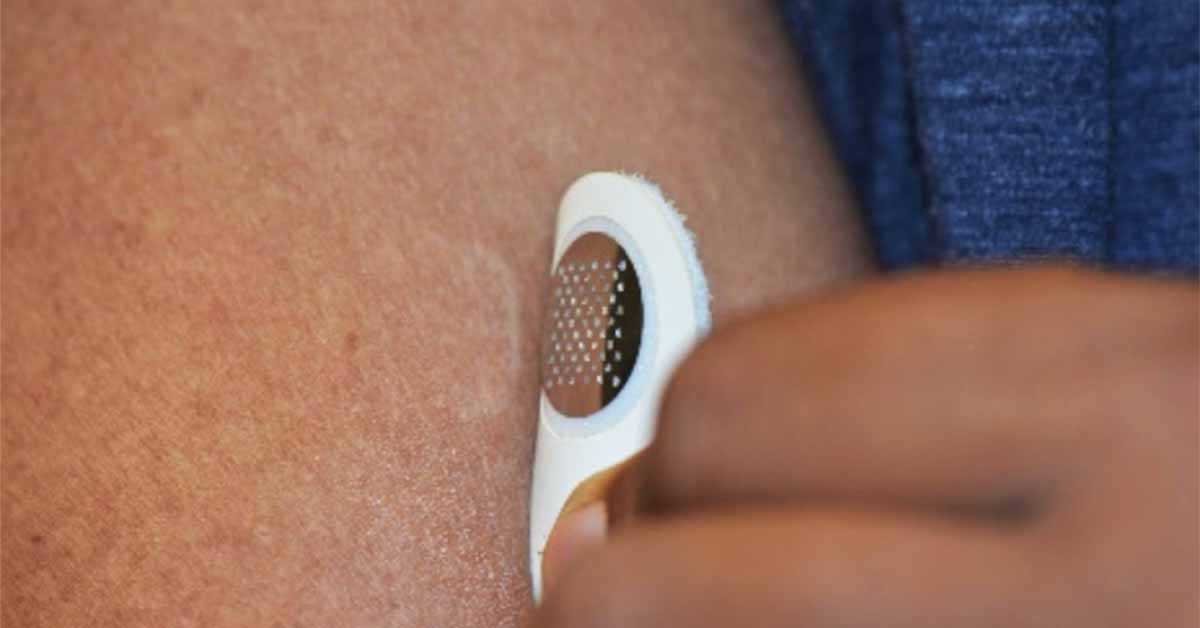
MOON101, which is based on Moonlight’s proprietary treatment platform dubbed TASIS — Targeted Allergen-Specific Immunotherapy within the Skin — involves the application of a small, minimally invasive “dermal stamp” that targets the delivery of allergen proteins to the immune cells in the top layer of skin, subsequently desensitizing the patient from a food allergy over time. The stamp is designed for at-home self-administration and is applied for only a few minutes and then discarded.
Said Samir Patel, Moonlight Therapeutics Co-founder and CEO:
The feedback from the FDA was encouraging and we will now prepare to submit our IND in order to evaluate the safety and tolerability of MOON101 in peanut-allergic individuals. Our first trial will include both adults and children with peanut allergy.
Said Dr Brian Vickery, Director of the Food Allergy Center at Emory and Children’s Healthcare of Atlanta:
Peanut allergy is a growing unmet medical need and affects over 6 million individuals in the United States alone. Over 1.5 million of those affected are children, which creates an incredible burden on families. As a result, there is a large demand from families for a safe, robust and convenient, treatment for peanut allergy.
- Microneedle Dermal Stamp to Treat Peanut Allergy Takes Another Step Forward — Company Press Release
- Investigational New Drug (IND) Application — US Food & Drug Administration
Take the 10-Second Quiz, Then Sign the Petition