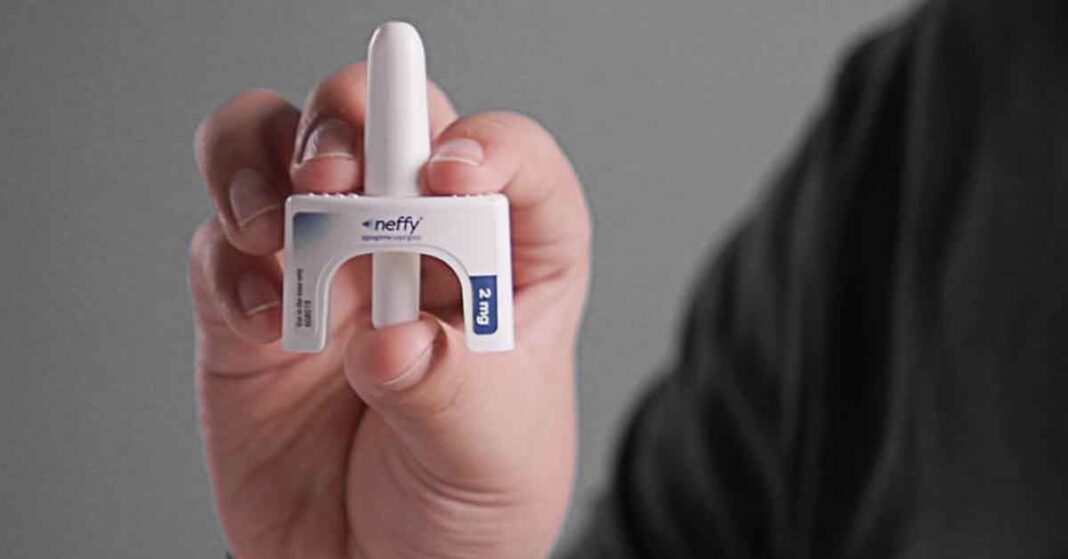
ARS Pharmaceuticals has announced their supplemental New Drug Application (sNDA) for a child dose of neffy has been accepted by the FDA and given priority review.
Neffy is the first needle-free emergency epinephrine device, FDA-approved for use in adults and older children 66 lbs and heavier in August 2024. Rather than jabbed in the thigh during a severe reaction, it is sprayed into a nostril, thus eliminating the fear of the needle many people harbor.
The new dose seeks approval for children 33-66 lbs.
Because the device was given priority review status, the FDA set the PDUFA date — the latest date at which they must decide whether to grant approval — at six months rather than ten. That date has been set for March 6, 2025.
ARS Pharma is also planning a dose for infants and toddlers weighing less than 33 lbs.
To learn more about neffy, see this interview with Richard Lowenthal, CEO of ARS Pharma, who answers your questions regarding the device’s heat tolerance, time-to-expiration, insurance coverage, maximum out-of-pocket cost, and many others: