For many years, treatment for peanut allergy focused primarily on avoidance and emergency management with epinephrine. While oral immunotherapy (OIT) has shown success in helping children become desensitized to peanuts, data on its effectiveness in adults has been limited. A recent phase II clinical trial, the Grown Up Peanut Immunotherapy (GUPI) study, sought to bridge this gap, and its findings offer a promising new path for adults living with this potentially life-threatening condition.
The GUPI study, a two-stage trial, investigated the efficacy of peanut OIT in adults with peanut allergies. Researchers enrolled 21 adults (8 female, average age 24.2 years) into an active treatment group. A separate, untreated control group was also recruited to help researchers understand the biological mechanisms behind the treatment.
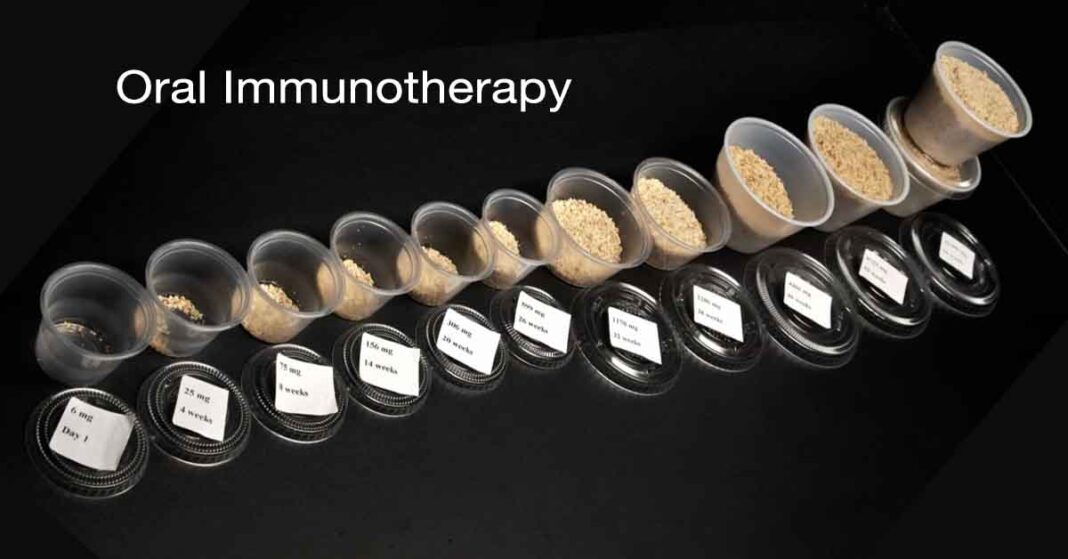
Before starting the OIT, all participants underwent a double-blind placebo-controlled food challenge (DBPCFC) to determine their baseline sensitivity to peanuts. The active treatment group then began a daily OIT regimen using real-world peanut products. They gradually increased their daily dose every two weeks until they reached a maintenance dose of 1000mg of peanut protein, roughly equivalent to four large peanuts.
The primary goal of the study was to see how many participants in the OIT group could tolerate a much larger dose of peanuts during a final food challenge at the end of the trial.
The results were encouraging: 67% of participants in the OIT group successfully reached the daily maintenance dose and met the study’s primary endpoint. The treatment led to a dramatic increase in the amount of peanut protein participants could safely consume. The median tolerated dose jumped from 30 mg (about 1/8th of a peanut) at the beginning of the study to 3000 mg (equivalent to 12 peanuts) at the final food challenge, a remarkable 100-fold increase in tolerance.
The study also highlighted improvements beyond just desensitization. OIT was associated with a noticeable improvement in quality-of-life measures for the participants. Furthermore, the study’s findings revealed key biological changes: those in the OIT group showed a reduction in peanut skin prick test sizes and an increase in peanut-specific IgG antibodies, which are associated with tolerance. These changes were not observed in the untreated control group, providing further evidence that the OIT was directly responsible for the positive outcomes.
While the results were overwhelmingly positive, the study did note some challenges. Three participants withdrew due to adverse reactions to the treatment, and another three left for reasons unrelated to the study. This highlights the need for continued research into safety and optimal protocols.
The GUPI study provides compelling evidence that peanut OIT is an effective treatment for adults with peanut allergies. While these findings are a significant step forward, the authors note that further research is needed to confirm the efficacy and better understand the safety profiles for different adult subgroups. The trial’s success, however, offers significant hope for millions of adults who currently manage their peanut allergy through constant vigilance and avoidance.