Home Search
viaskin peanut - search results
If you're not happy with the results, please do another search
Non-Profit Institute to Review Benefits of Commercial, Private Practice Immunotherapies for Peanut Allergy
An independent review of the value, costs and effectiveness of Viaskin Peanut, AR101, and private practice peanut OIT.
Milk and Peanut Clinical Trial Advances
Announcements regarding two food allergy therapies that are in or advancing toward clinical trials were made yesterday.
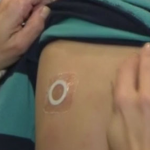
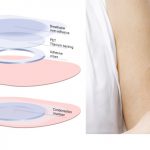
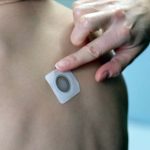
DBV Completes Phase III Study of Peanut Patch
The phase III trial was designed to assess the safety and routine clinical use of Viaskin Peanut.
Peanut Patch Phase III Enrollment Ends, Results Expected Toward End of 2017
The study received higher-than-expected patient demand.
Study: Peanut Immunotherapy Significantly Reduces Risks Due to Cross-Contact in Common Packaged Foods
The study model used common packaged foods such as cookies, ice cream, and doughnuts as references.
Viaskin Milk Patch Receives Positive Recommendation to Proceed to Phase II
The board overseeing the Phase I study of the milk allergy therapy found no safety concerns.
Peanut Patch Receives FDA's Breakthrough Therapy Designation
The Viaskin Peanut patch received the BT designation after a positive Phase IIb trial.
Private Law Firm to Investigate Claims on Behalf of Investors in DBV Technologies
It is unknown how this development will affect the ability of the company to eventually offer Viaskin Peanut as an FDA approved therapy.
SnackSafely.com Newsletter: 2019/08/08
DBV Technologies Submits Biologics License Application to FDA for Viaskin Peanut for the Treatment of Peanut Allergy (+4 Articles)
DBV Technologies Announces Positive 3-Year Results from EPITOPE Phase 3 Open-Label Extension Study
68.2% of subjects completed the oral food challenge (~12-14 peanut kernels) without meeting stopping criteria, compared to 30.7% at month 12.