Latest Food Allergy News
Find Allergy-Friendly Products
Sponsored Epinephrine
neffy® Commercial Insurance Coverage Update as of May 19, 2025
Sign up to receive updates using the included form.
From 23 Allergies to Food Freedom: Isaiah’s Journey with TIP
His journey highlights the challenges he overcame and offers valuable advice for other teens considering TIP treatment.
A Conversation About the Food Allergy Science Initiative and Their Cutting-Edge Research
If you were wondering whether food allergy research was making progress, watch this video and wonder no more.
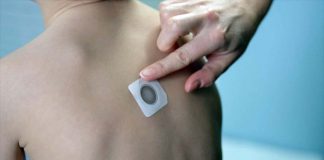
Optimism Grows for Toddlers with Peanut Allergies as Viaskin Patch Safety Study Begins
DBV Technologies anticipates their submission to the FDA will occur in the second half of 2026
Labeling Insights
When a Manufacturer Says ‘Trust Us’ Regarding Allergens
They're not REQUIRED to disclose the possibility of cross-contact with allergens. Should you trust them to?
Coupons
Coupons and Offers
Coupons and discounts for your favorite allergy-friendly foods! Visit snacksafely.com/coupons.
Manufacturer Partnership
SnackSafely.com Manufacturer Partnership
Our growing partnership of companies dedicated serving the food allergy and celiac communities with transparency.