With the demise of the Auvi-Q, competition in the auto-injector space has all but evaporated in the US leading to a dearth of choices and higher prices.
Back in December, we published an article about Windgap Medical’s innovative auto-injector design which had just been awarded US patent 9199037, entitled “Portable drug mixing and delivery system and method“. The patent shed some light on Windgap’s plans to deliver a smaller device that mixes wet and dry components when activated, thus promising temperature stability and a longer shelf-life.
We talked to the company’s CEO, Christopher Stepanian, to find out more about the product that Windgap is developing that seeks to “improve the quality of life for patients living with severe allergies.”
SnackSafely.com: Chris, thanks for your time. Tell us a bit about yourself, your education, and your experience.

Stepanian: My background is in engineering, composites and nanomaterials, and business. I’ve a BS from RPI [Rensselaer Polytechnic Institute], an MS from Texas A&M, both in engineering, and an MBA from the MIT Sloan Fellows Program. During my career I’ve worked in aerospace, automotive, contract research, and insulation industries. I’ve had roles in R&D, operations, finance, marketing, and strategy. I’ve been a guy thinking up new ideas, making them work in manufacturing, and figuring out how to build a business selling them.
SnackSafely.com: What was the genesis for you and your colleagues to design a better auto-injector?
Stepanian: Many startups invent a technology and try to figure out who needs it – “A hammer in search of a nail.” We started with the nail, a need.
Patients and allergists were telling us that some of their desires were unmet. Patients were looking for something that was easier to carry, thermally stable, and easy-to-use. Allergists that we contacted were seeking higher patient compliance since an epinephrine auto-injector only works when you have it with you and you use it when needed. It resulted in a lot of conversations and ideas from which our ANDI auto-injector was created. [ANDI is Windgap’s working name for the device, which stands for Automatic recoNstitution Dual-chamber Injector.]
The ah-ha moment was at the Harvard Square MBTA station when my co-founder Brent [Brent Buchine] and I were heading home. I asked him if a dry form of the drug could be reconstituted by some of the microfluidic mixers that he and I were discussing… and Windgap began.
SnackSafely.com: Once you decided on this project, how did you go about assembling your team?
Stepanian: The team formed slowly for us at Windgap. We stayed virtual with just founders until we raised our first equity round of financing, led by Launchpad – an angel investment group – here in Boston. After that Brent and I hired Adam [Adam Standley], our Director of Product Development, who was a consultant for the company of many months beforehand, helping to develop ANDI’s core technology. After our second round of financing, led by GRT BioEdge Ventures, we hired our VP of Operations, Evan [Evan Sherr], since we were transitioning from bench-scale prototypes to devices that had to be manufacturable at high-volumes, with appropriate quality.
SnackSafely.com: Tell us about the design phase and how you arrived at a working prototype.
Stepanian: With our first outside round of equity financing, we took the opportunity to take a step back. We worked with an outside medical device design firm to examine how our core patient and device needs could be satisfied.
We examined dozens of approaches and settled upon three that were fleshed out in some detail. We down-selected to two designs, prototyped and tested key portions of the device designs, and made a final selection. That final selection we turned into a handful of working auto-injectors which we then tested to confirm that they met all of our initial product requirements.
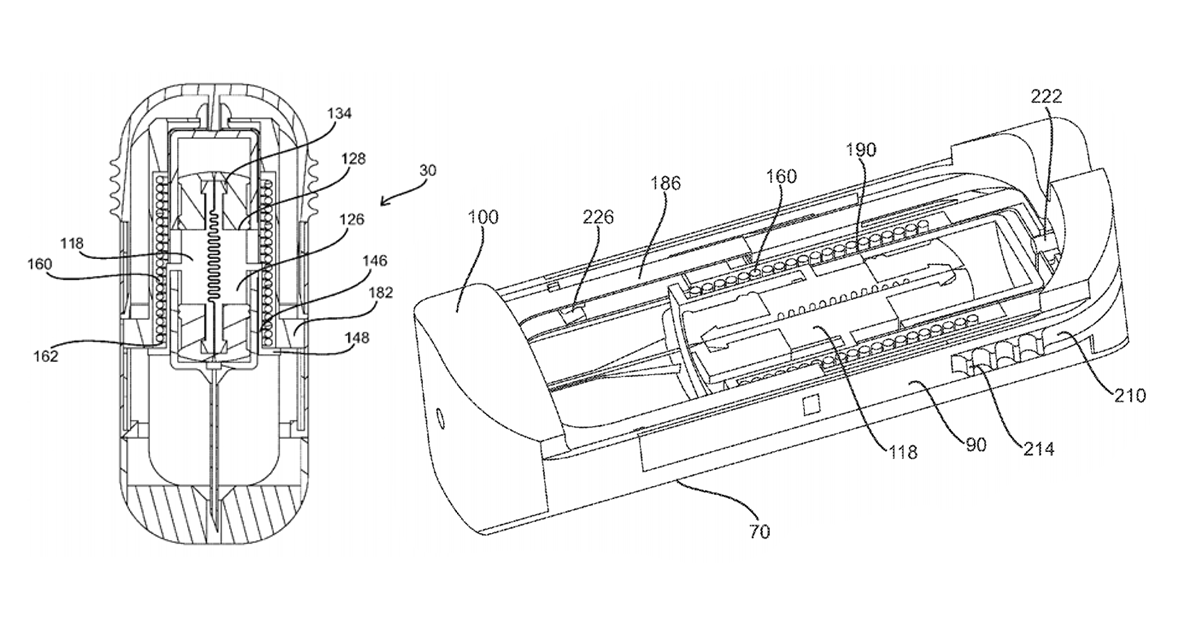
SnackSafely.com: Describe your patent and what makes your design unique.
Stepanian: We now have two patents and six additional patent applications have published on the USPTO website with dozens more pending!
The focus of all of our published patents is on the idea of facilitating wet/dry mixing in the drug delivery space. Our goal is to enable the reconstitution or mixing of drugs in a controlled way in a very small volume. Our approaches are focused on situations where the stability of a drug might be an issue and ease-of-use is a concern.
SnackSafely.com: What consumer input did you solicit during your design and refinement process? How did you incorporate that feedback?
Stepanian: We started with that feedback early-on in our company. Surveys and informal conversations were how we learned about the market and both the patient’s and the care-giver’s needs. There’s quite a bit of peer-reviewed literature out there in the US and internationally and have had the opportunity to read a bunch of it. Brent and I have demonstrated our training device for patients and allergists and then we listened to their feedback. This happened as we worked to establish our initial design and has helped us to direct it as the product continues to develop.

SnackSafely.com: Describe the steps toward getting your product to market that have been accomplished to date.
Stepanian: We first addressed questions of physics – is the dry drug temp-stable and can it be quickly reconstituted? We then moved to questions of engineering – can a prototype be designed and machined that reconstitutes our drug formulation and can inject it into a test machine?
Now we’re addressing questions of manufacturability – can we make devices using injection-molded plastics that are representative of what we believe is a manufacturable and high-quality design, assembled off of a pilot production line. It’s a challenge to knit together any supply chain, let alone one that has the quality and documentation requirements of a combination (pharmaceutical/device) product.
SnackSafely.com: What steps remain before we can purchase your auto-injector at our local pharmacy?
Stepanian: We still need to make our next generation of molds, produce registration lots, put those lots on long-term stability test, generate test data, put together our submission to the FDA and await their response. We anticipate submitting to the FDA for consideration in 2018 and anticipate a response from the Agency within a year.
Windgap is evaluating go-to-market partnership opportunities with a number of pharmaceutical companies with an allergy focus. This partner will provide Windgap with the resources that are needed to commercialize our product and reach the up to 28 million patient potential addressable market in the US.
SnackSafely.com: What else should the community know about Windgap?
Stepanian: We are, above all, a small business. We’re working very hard to bring a complex device and supply chain together to turn our ideas into reality. The team is small but incredibly capable, dedicated and versatile.
We have been well-supported by our investors like Launchpad and GRT BioEdge Ventures, our Board, our advisors, and our supply-chain partners along with the Mass Life Sciences Center and their Accelerator Loan that has helped us speed our time-to-market.
It’s challenging to bring a life-saving product like an epinephrine auto-injector to market. However, I personally believe that the millions of potential users of epinephrine auto-injectors would benefit from a product that is positively differentiated from those marketed today. When we see our first customer carrying our device, it will make all of the late nights worthwhile.






The current Epipen is quickly becoming archaic, with its size and cost. Its nice to see someone go toe to toe with a more convenient size.