An epinephrine nasal spray alternative to auto-injectors for anaphylaxis has taken the final step forward toward approval in the EU.
ARS Pharmaceuticals — the company behind “Neffy”, the commercial name for their ARS-1 epinephrine nasal spray — announced today that the Marketing Authorization Application (MAA) they filed was accepted by the European Medicines Agency.
Acceptance of an MAA, which is similar to a New Drug Application (NDA) in the US, indicates the agency is satisfied with the completeness of the application and will begin consideration for approval. The company has yet to submit its NDA to the Food and Drug Administration.
Intranasal delivery of emergency epinephrine would provide allergic consumers a needle-free option and could spur increased use of the drug in anaphylactic emergencies leading to better outcomes.
Here follows the ARS press release in its entirety. Click here for more of our coverage of Neffy/ARS-1.
ARS Announces Acceptance of Market Authorization Application submission to European Medicines Agency for Neffy (ARS-1; epinephrine nasal spray)
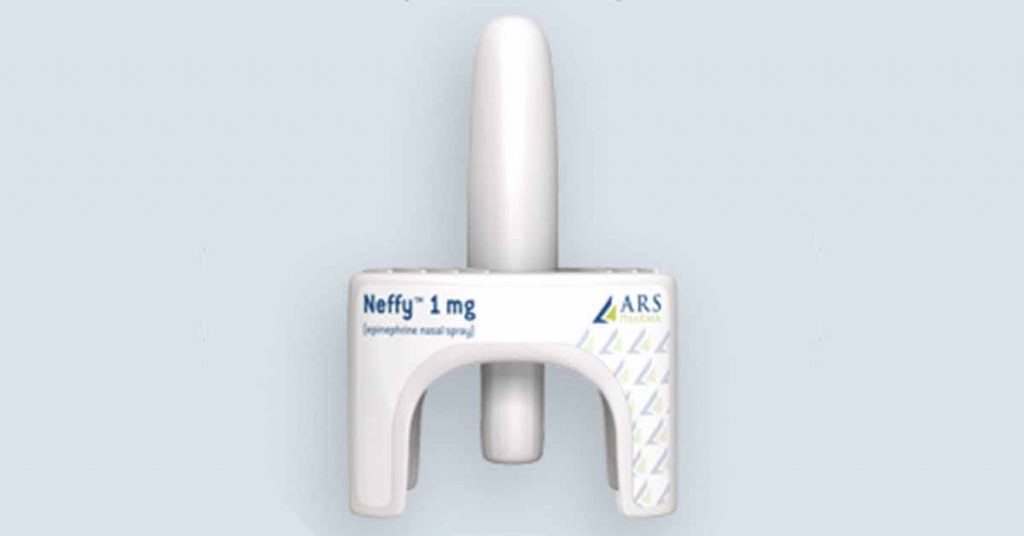
SAN DIEGO, Nov. 30, 2020 /PRNewswire/ — ARS Pharmaceuticals (ARS) announced today that the European Medicines Agency (EMA) has accepted a Marketing Authorization Application (MAA) submission for review of NeffyTM (ARS-1; epinephrine nasal spray) an epinephrine nasal spray for the emergency treatment of severe allergic reactions, including anaphylaxis. This follows ARS’ recent announcement of an exclusive licensing agreement with Recordati for commercialization of Neffy in 93 countries including those in the European Union. Neffy is the trademark name established in the U.S.; the European product name has not yet been established.
“This first of our anticipated global regulatory submissions is an important milestone for Neffy and for the millions of people in Europe living with life-threatening allergies,” said Richard Lowenthal, President and Chief Executive Officer of ARS Pharmaceuticals. “We look forward to working together with the EMA and our marketing partner Recordati to make this simple to use, rapidly administered and needle-free way to treat severe allergic reactions available for patients and help reduce the risks of anaphylaxis.”
The MAA submission to EMA includes data from multiple clinical studies showing that 1 mg of Neffy achieves epinephrine exposures that are similar to a 0.3 mg epinephrine IM injection or EpiPen, with rapid absorption (time to peak plasma levels) and clinical response based on surrogate endpoints. Because of its innovative delivery method, Neffy has the potential to be as effective as injections in the treatment of severe allergic reactions in a more convenient and less intimidating delivery device. It’s needle-free, small and easy-to-use delivery system may help eliminate anxiety and overcome hesitation that is common with injectable epinephrine. A dosage strength of 0.65 mg is under development for children weighing between 15kg and 30kg and is expected to be filed shortly after the initial approval.
In Europe, based on epidemiology data, about 4% of the general population has experienced an anaphylactic episode. Overall annual net sales of epinephrine auto-injectors in Europe are around $120 million USD based on IQVIA prescription data, representing less than 10% of the eligible population. According to the European Anaphylaxis Registry, less than 15% of anaphylaxis episodes are self-treated with an auto-injector. The introduction of Neffy would be a welcome new tool for more patients with severe allergies to safely, quickly and painlessly administer lifesaving epinephrine.
About ARS Pharmaceuticals, Inc.
ARS Pharmaceuticals is dedicated to empowering at-risk patients and caregivers to better protect themselves from severe allergic reactions that could lead to anaphylaxis. The Company is developing Neffy (ARS-1), an intranasal epinephrine spray with a unique absorption technology that could be easy-to-use, needle-free, convenient and more reliable for patients and loved ones at-risk of severe allergic reactions to food, medications and insect bites that could lead to life-threatening anaphylaxis. For more, visit www.ars-pharma.com.
About Recordati
Recordati, established in 1926, is an international pharmaceutical group, listed on the Italian Stock Exchange (Reuters RECI.MI, Bloomberg REC IM, ISIN IT 0003828271), with a total staff of more than 4,300, dedicated to the research, development, manufacturing and marketing of pharmaceuticals. Headquartered in Milan, Italy, Recordati has operations throughout the whole of Europe, Russia, Turkey, North Africa, the United States of America, Canada, Mexico, some South American countries, Japan and Australia. An efficient field force of medical representatives promotes a wide range of innovative pharmaceuticals, both proprietary and under license, in a number of therapeutic areas including a specialized business dedicated to treatments for rare diseases. Recordati is a partner of choice for new product licenses for its territories. Recordati is committed to the research and development of new specialties with a focus on treatments for rare diseases. Consolidated revenue for 2019 was € 1,481.8 million, operating income was € 465.3 million and net income was € 368.9 million.





