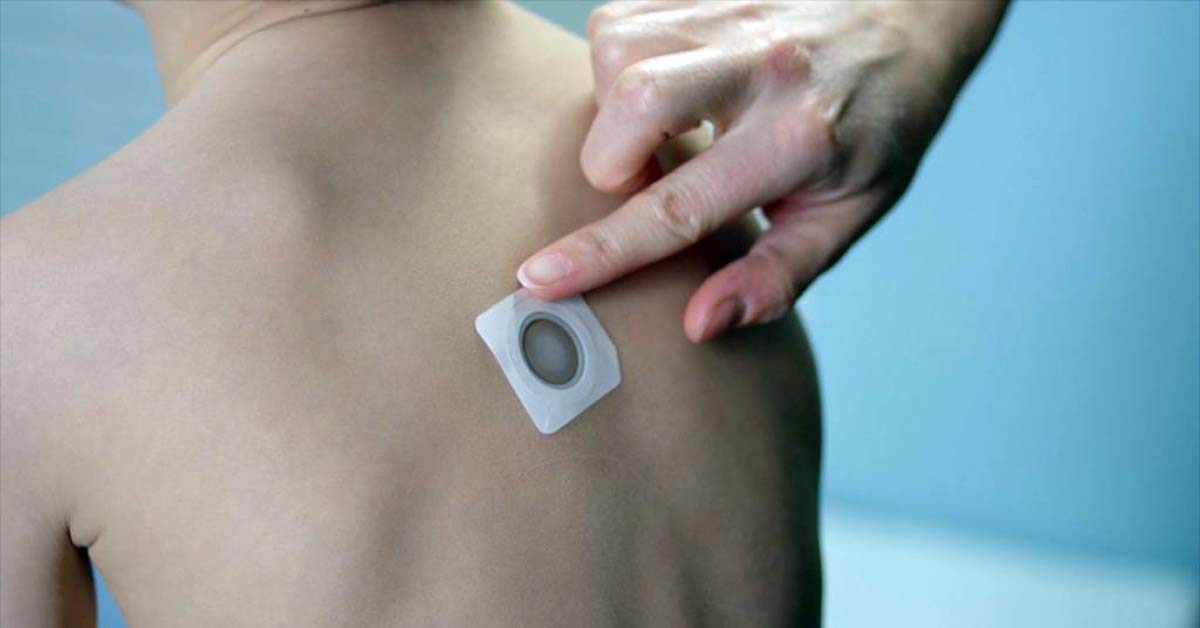
DBV Technologies, the French biopharma company behind the Viaskin Peanut epicutaneous immunotherapy patch to treat peanut allergy, has received feedback from the US Food and Drug Administration (FDA) that it believes provides a clear path to FDA approval.
Back in August of 2020, the FDA denied market approval for Viaskin Peanut, voicing concerns that adhesion of the patch would affect the efficacy of the therapy and indicating the need for modifications and a new human factor study.
Then, two weeks ago, the company announced it was laying off two thirds of its staff.
Since then, the company has met with the FDA and believes that the feedback received from the FDA provides a well-defined regulatory path forward. In exchanges with the FDA, DBV proposed potential resolutions to two main concerns identified by the FDA in the CRL: the impact of patch adhesion and the need for patch modifications. DBV will address details about a new human factor (HF) validation study and additional chemistry, manufacturing and controls (CMC) data in subsequent interactions with the FDA.
The FDA agreed with DBV’s position that a modified Viaskin™ Peanut patch should
not be considered as a new product entity provided the occlusion chamber of the
current Viaskin™ Peanut patch and the peanut protein dose of 250 µg
(approximately 1/1000 one peanut) remains unchanged and performs the same
way it performed previously.
Said Daniel Tassé, Chief Executive Officer of DBV Technologies:
We are very encouraged by the positive feedback received from the FDA, and we appreciate the clarity provided. I want to thank the DBV team for their dedication in working to address the FDA’s findings over the past few months. We intend to advance a remediation plan for Viaskin™ Peanut and work closely with FDA to review protocols and re-file our BLA as soon as possible, so that we can bring Viaskin™ Peanut, if approved, to patients suffering from peanut allergies.
Said Dr Pharis Mohideen, Chief Medical Officer of DBV Technologies:
We look forward to working with our investigators, clinical trial sites, and key stakeholders as we continue in our development of investigational Viaskin™ Peanut. Everyone at DBV remains highly committed to the possibility of bringing this innovative treatment option to patients and families.
Viaskin therapies for milk and egg allergies are in DVB’s development pipeline.