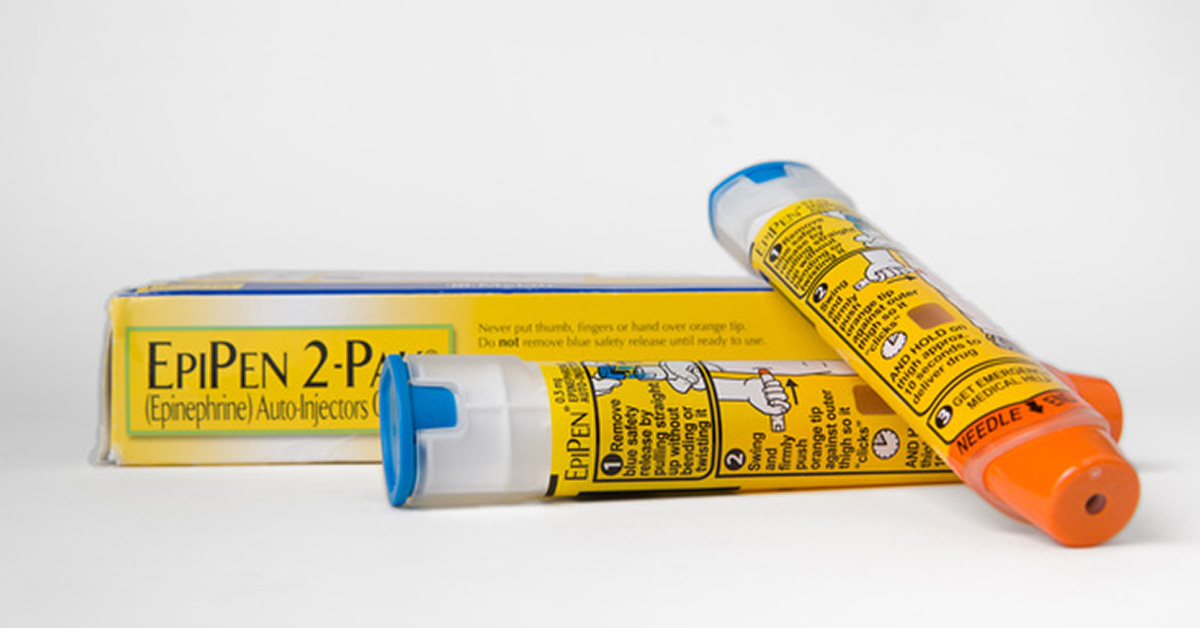
As we reported yesterday, Spectrum Laboratory Products issued a worldwide recall of certain lots of their powdered epinephrine used to manufacture finished dose products that treat a variety of life-threatening conditions including anaphylaxis.
Although some number of hospitals, clinics, and physicians’ offices will no doubt be affected, it is unknown whether epinephrine auto-injectors will be affected by the recall. We reached out to the manufacturers of auto-injectors marketed in the US — Viatris (formerly Mylan for EpiPen, EpiPen Jr), Kaleo (Auvi-Q), Amneal (Adrenaclick generic), Teva (EpiPen generic) and Adamis (Symjepi) — to find out.
The first to respond — Lauren C Kashtan, Head of Global Corporate, Portfolio and Operations Communications for Viatris — states the EpiPen family of auto-injectors is NOT affected by the recall:
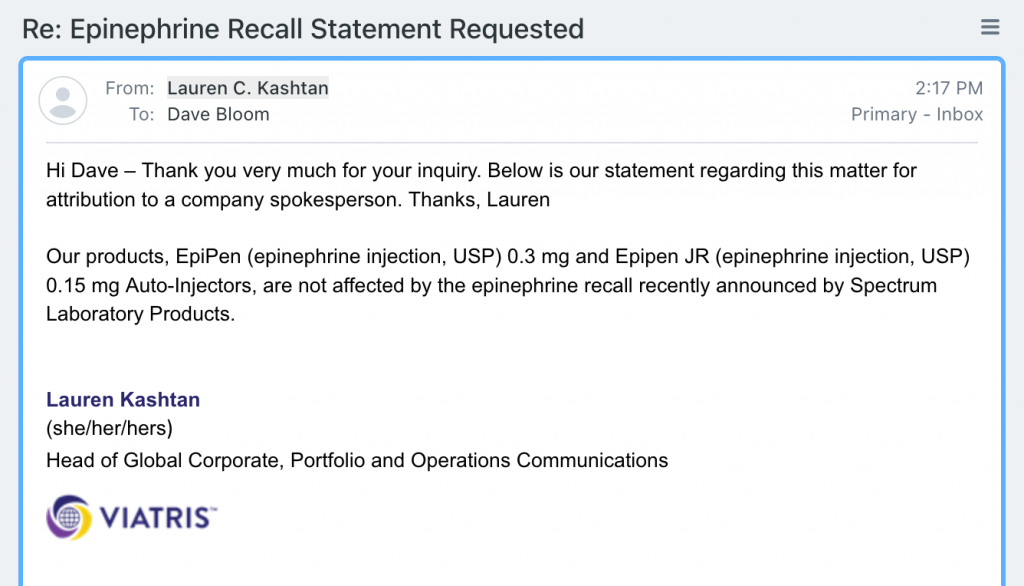
Our thanks to Ms Kashtan for her prompt reply which will put the minds of many owners of EpiPens at ease.
Click here to see Kaleo’s response regarding Auvi-Q.
We will post the statements of the remaining manufacturers as we receive them.