Home Search
omalizumab - search results
If you're not happy with the results, please do another search
Omalizumab (Xolair) Is Superior to Oral Immunotherapy in Multi-Food Allergy Treatment
Research finds food allergic patients have better outcomes with omalizumab than oral immunotherapy.
Study: Omalizumab (Xolair) May Help Treat Idiopathic Anaphylaxis
Small study finds drug may be effective in treating IA.
Study: Treatment with Omalizumab Raises Tolerance in Patients with Severe Food Allergies
Patients reached full tolerance for 70.4% of the tested foods which were reintroduced to the patients’ diet without the need for OIT.
Asthma drug shows promise in blocking food allergy reactions
Study finds a new biological trigger for anaphylaxis and a drug that stops it.
Study Suggests Biologics Safe to Recommend for Treatment of IgE-Mediated Food Allergy
The research team emphasized that biologics, whether used alone or combined with OIT, are an advanced method for managing food allergies.
A Conversation About Mia, a 9-Year-Old Undergoing Xolair Therapy for Food Allergies
Ilana Dubrovsky discusses the progress of her nine-year-old daughter, who is currently undergoing therapy.
FDA Approves LongBio’s Anti-IgE Antibody for Clinical Trials to Treat Food Allergy
Results to be presented at AAAAI meeting in February.
Are Biologics the Future of Food Allergy Treatment? One Study Weighs Pros and Cons
UNC School of Medicine researchers delve into the perspectives of community and academic providers on the role of biologics and food allergy.
Bacon Safe for Alpha-Gal? Peanuts Safe for Peanut Allergy? GMOs in the News
Genetically modified alternatives are here, like it or not.
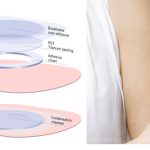
Viaskin Patch Improves Treatment for Pediatric Milk Allergies
The patch releases small doses of a milk allergen protein through the skin without ingestion.