Home Search
viaskin - search results
If you're not happy with the results, please do another search
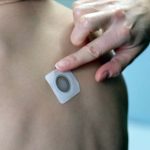
Optimism Grows for Toddlers with Peanut Allergies as Viaskin Patch Safety Study Begins
DBV Technologies anticipates their submission to the FDA will occur in the second half of 2026
DBV Confirms Alignment with FDA on Accelerated Approval Pathway for the Viaskin® Peanut Patch in...
Toddlers study on-track to initiate in 2Q 2025.
DBV Announces Positive Regulatory Updates for the Viaskin® Peanut Patch in the United States and...
Company to pursue an Accelerated Approval pathway for toddlers ages 1–3 years-old.
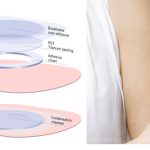
Viaskin Patch Improves Treatment for Pediatric Milk Allergies
The patch releases small doses of a milk allergen protein through the skin without ingestion.
Viaskin Milk Results Shows Potential for Treatment of Milk Allergy in Children
95.5% of the participants completed treatment, with most adverse effects being mild or moderate reactions at the application site.
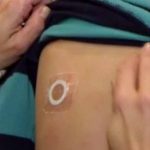
DBV Announces Promising 2-Year Results from Ongoing Viaskin “Peanut Patch” Toddler Study
81.3% of subjects who completed the oral food challenge reached an eliciting dose of ≥1,000 mg after 24 months of treatment.
DBV Outlines Path to Regulatory Approval of Viaskin Peanut Patch
Are Viaskin therapies finally on the horizon?
FDA Issues Partial Hold on DBV Viaskin Peanut Patch Trial
Another delay for the long-awaited therapy.
DBV Begins Phase 3 Study of Redesigned Viaskin Peanut Patch in Children Ages 4-7
Viaskin is testing a new version of their patch designed to address the FDA's concerns.
DBV Announces Positive Topline Results from Phase 3 Trial of Viaskin Peanut in Peanut-Allergic...
Viaskin Peanut demonstrated a statistically significant treatment effect with 67.0% of subjects meeting the treatment responder criteria after 12 months.