Home Search
viaskin - search results
If you're not happy with the results, please do another search
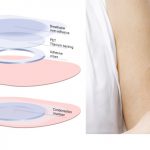
Viaskin Peanut, Milk and Egg Patch Therapies: Promising News from the AAAAI Conference
We are cautiously optimistic that a safe, effective, easy to administer treatment for food allergy may be on the horizon.
Viaskin Milk Patch Receives Positive Recommendation to Proceed to Phase II
The board overseeing the Phase I study of the milk allergy therapy found no safety concerns.
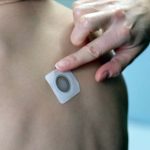
Peanut Patch Treatment Continues to Help Toddlers Safely Build Tolerance Over Three Years
After three years of treatment, more than 70% could tolerate about three to four peanut kernels.
A new era in food allergy treatment has begun
Innovative treatments aim to do more than manage symptoms; they offer the potential for long-term remission and even a cure.
Newsletter Archive
Catch up on past newsletters.
Children’s Response to Peanut Patch Therapy Increased Over 60 Months
VP250 may lead to an accumulation of clinical benefit with high treatment compliance, making this a valuable option for children with allergies.
DBV Technologies Announces Positive 3-Year Results from EPITOPE Phase 3 Open-Label Extension Study
68.2% of subjects completed the oral food challenge (~12-14 peanut kernels) without meeting stopping criteria, compared to 30.7% at month 12.
FDA Removes Hold Allowing DBV’s Pivotal Phase 3 Peanut Patch Trial to Commence
The phase 3 trial of the modified Viaskin Peanut patch has been cleared for commencement.
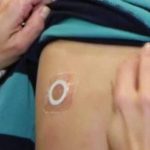
Skin Patch Immunotherapy Determined Safe and Effective in Toddlers With Peanut Allergy
According to research presented at this year's #ACAAI22 meeting.
Private Law Firm to Investigate Claims on Behalf of Investors in DBV Technologies
It is unknown how this development will affect the ability of the company to eventually offer Viaskin Peanut as an FDA approved therapy.