Fact: there are currently no approved peanut allergy treatments for children younger than four years old.
Research presented at this year’s American College of Allergy, Asthma & Immunology (ACAAI) 2022 annual meeting shows that children 1-3 years old with peanut allergy who received epicutaneous immunotherapy showed a statistically significant response to treatment.
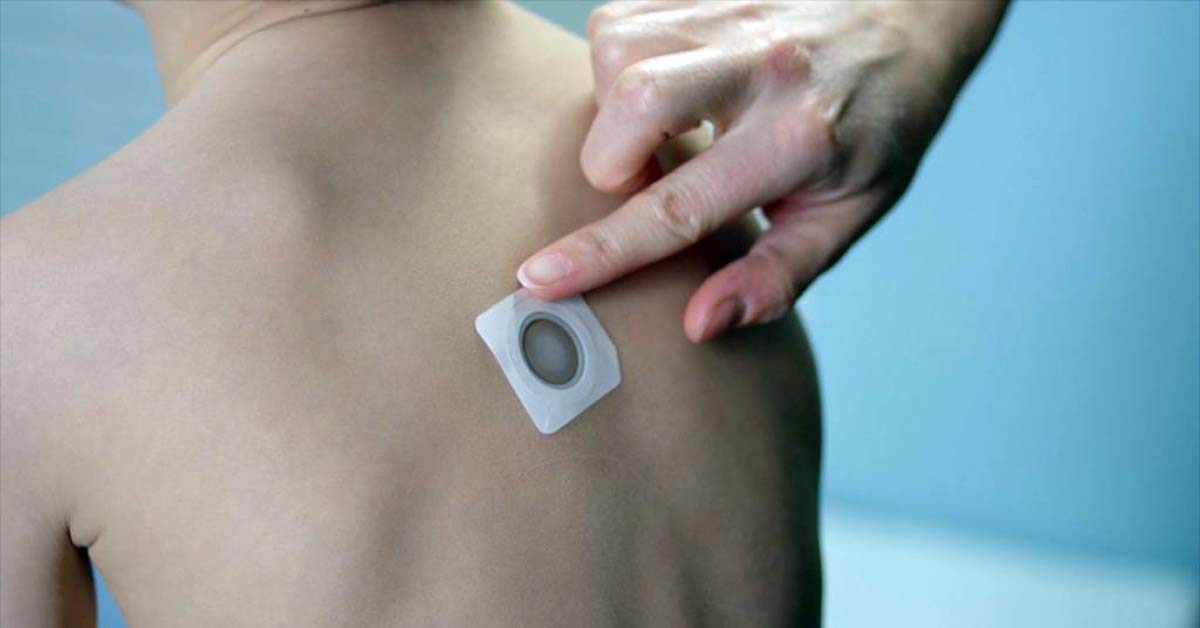
Epicutaneous immunotherapy (EPIT) exposes tolerance-promoting immune cells in the skin to a dermal patch containing a tiny dose of a food protein. DBV Technologies is pioneering work in EPIT with their Viaskin Peanut therapy.
During a phase 3 clinical trial known as “EPITOPE”, researchers sought to determine the safety and efficacy of EPIT in children ages 3 and younger. Candidate children were administered a double-blind, placebo-controlled food challenge (DBPCFC) to determine whether they could tolerate a 300mg eliciting dose or less of peanut protein equivalent to 1½ peanuts. Those that could not were randomly assigned to cohorts to receive either a peanut patch for 12 months of daily treatment (244 children) or a placebo patch (118 children). Safety was evaluated based on treatment-emergent adverse events (TEAEs).
Of the 362 children (median age: 2.5 years; 68.8% male), 84.8% were able to complete treatment. About 67% of children in the active peanut patch cohort and 33.5% in the placebo patch cohort achieved the primary efficacy endpoint of tolerating 300mg of peanut protein. In addition, 64.2% of children who received the active patch and 29.6% who received the placebo had a peanut protein eliciting dose of 1000mg or greater.
Most TEAEs were mild or moderate reactions confined to the application site. Serious TEAEs occurred in 8.6% of children using the peanut patch and in 2.5% using the placebo patch. Among the children using the peanut patch, 4 (1.6%) suffered treatment-related anaphylaxis and 8 (3.3%) discontinued participation due to a TEAE.
The researchers concluded:
Twelve months of epicutaneous immunotherapy with a patch containing 250µg peanut protein was associated with a statistically significant response vs placebo among peanut-allergic children aged 1-3 years.
- EPITOPE study results: phase 3, randomized, double-blind, placebo-controlled study of epicutaneous immunotherapy in peanut-allergic toddlers — Annals of Allergy, Asthma & Immunology
- Epicutaneous Immunotherapy Safe, Effective in Toddlers With Peanut Allergy — Pulmonology Advisor
- Epicutaneous Immunotherapy (EPIT) — FARE