Kaléo and the pan-Canadian Pharmaceutical Alliance (pCPA) Complete Negotiations for ALLERJECT® (epinephrine injection, USP) Auto-Injector for the Treatment of Serious Allergic Reactions Across Canadian Provinces
- Kaléo and the pan-Canadian Pharmaceutical Alliance (pCPA) complete negotiations for ALLERJECT® (epinephrine injection, USP) auto-injector
- Ontario* and Quebec* public drug plans are first to list ALLERJECT with other provinces expected to follow soon
- ALLERJECT is also covered by the largest private insurance plans across Canada
RICHMOND, Va., Aug. 10, 2020 /CNW/ – Kaléo, a privately held U.S. pharmaceutical company, today announced that it has completed negotiations with the pan Canadian Pharmaceutical Alliance (pCPA) for a Letter of Intent regarding ALLERJECT® (epinephrine injection, USP) auto-injector for the treatment of serious allergic reactions. In addition to these public programs, the largest private insurance plans, covering more than eighteen million Canadians, now also list ALLERJECT as a covered benefit.
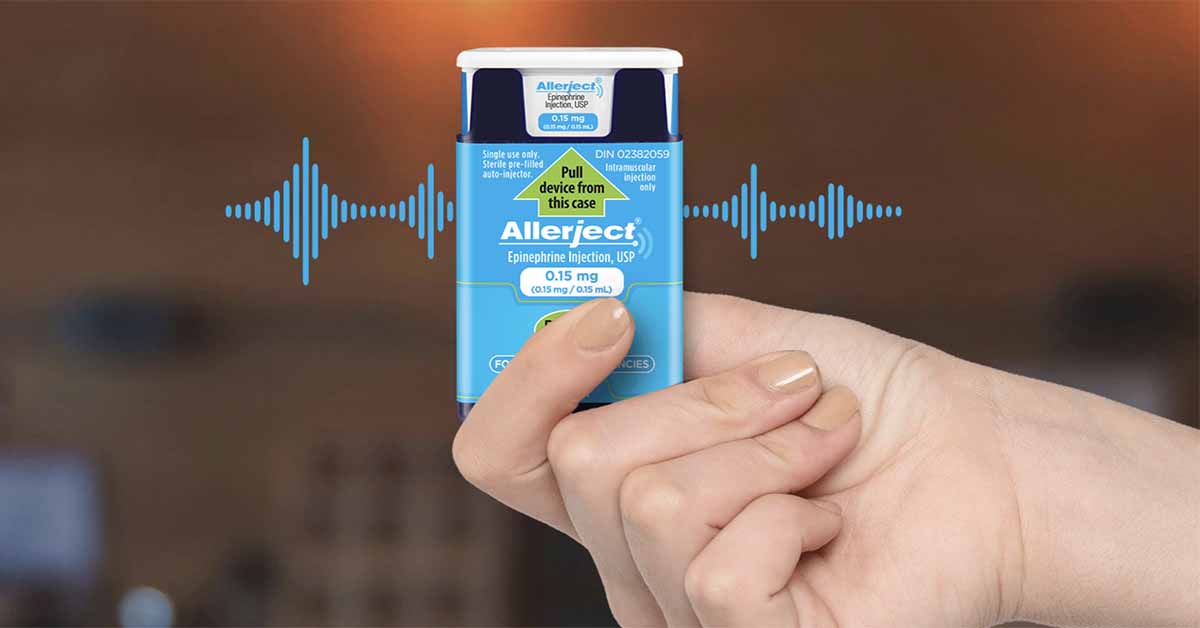
ALLERJECT is a portable epinephrine auto-injector with an innovative voice instruction system that helps guide patients and caregivers step-by-step through the injection process and has a retractable needle system. ALLERJECT is available in a 0.15 mg dose (for patients who weigh 15 to 30 kg (33 lbs to 66 lbs)) and a 0.3 mg dose (for patients who weigh 30 kg or more (66 lbs or more)).
“This is welcome news for the more than thirteen million Canadians1 who rely on public insurance to access their prescription medications,” said Omar Khalil, general manager of allergy and pediatrics at kaléo. “The Ontario and Quebec public drug plans were the first to list ALLERJECT with additional provinces expected to follow soon. We are excited this will allow patients and families the option to access ALLERJECT for their epinephrine auto-injector needs.”
ALLERJECT is indicated for the emergency treatment of serious allergic reactions (anaphylaxis) and is intended for people who are at risk and for people with a history of serious allergic reactions (anaphylaxis). Anaphylaxis is the term for a severe, life-threatening allergic reaction that some people have to foods (like peanuts and shellfish), insect stings, certain medicines, latex, or other allergens. These reactions can also be triggered by exercise or even by unknown causes. A severe allergic reaction occurs when a person is exposed to an allergen (an allergy-causing substance). When the allergen enters the body, it triggers the release of chemicals that can lead to life-threatening symptoms.2
“It is so important that every Canadian at risk of anaphylaxis has affordable and consistent access to an epinephrine auto-injector,” said Jennifer Gerdts, executive director of Food Allergy Canada. “The news that ALLERJECT will be broadly covered increases the access patients have to a device, helping to ensure they are prepared in case of a serious allergic reaction. Understanding what to do in case of an allergic emergency is particularly important as families are getting ready for back-to-school and reviewing their Anaphylaxis Emergency Plans.”
As many as 2.6 million Canadians may have at least one food allergy.3 Up to 740,000 people are estimated to be at risk for anaphylaxis due to food or insect stings alone at least once in their lifetime.1 A potentially life-threatening allergic reaction can happen anywhere – and can happen quickly4-6– reinforcing the importance of patients, families and caregivers having timely and reliable access to an epinephrine auto-injector.
ALLERJECT became widely available in pharmacies across Canada on May 19, 2020. Patients interested in obtaining ALLERJECT should speak to their healthcare provider and are encouraged to check with their local pharmacy for additional availability and pricing details.
For more information, please visit www.Allerject.ca.
*Ontario: Coverage under the Ontario Public Drug Programs is available for patients with a valid prescription for ALLERJECT
*Quebec: Codes assigned by RAMQ (Official Mark of the Régie de l’assurance maladie du Québec) are as follows: 0.15 mg is 98069 and 0.3 mg is 98070
About Anaphylaxis7,8
Anaphylaxis (pronounced anna-fill-axis) is the most serious type of allergic reaction. During anaphylaxis, a person may have trouble breathing or experience a drop in blood pressure. These symptoms can lead to death if not treated. Health Canada, the Canadian Food Inspection Agency (CFIA), allergy associations, and the medical community have identified the key substances most frequently associated with food allergies and allergic-type reactions, including: eggs, milk, mustard, peanuts, crustaceans and molluscs, fish, sesame seeds, soy, sulphites, tree nuts, wheat and triticale.
The FDA’s Recent Guidance Has Made Ingredient Labeling Less Reliable. Here’s How SnackSafely.com Can Help