Home Search
ar101 - search results
If you're not happy with the results, please do another search
Phase II Trials of AR101 with Dupilumab for Peanut Allergy to Begin
Study scheduled to commence next week.
Aimmune Issues Update on AR101 Peanut Oral Immunotherapy Candidate
The company announces their timeline for continued progress toward an FDA approved standardized therapy.
OIT Drug That Treats 15 Food Allergies Increased Eliciting Threshold but Missed Primary Endpoint
Candidate warrants further clinical investigation.
Nestlé Acquires Aimmune, Partners with Enterome to Develop Food Allergy and IBD Candidate
Nestlé, the consumer products powerhouse, is expanding its pharmaceutical portfolio with massive investments.
Study: More Than 80% of Patients Could Tolerate 2000mg of Peanuts after 2 Years...
Safety and efficacy increased over time with treatment.
Aimmune Presents New Clinical Data From Patients Treated With PALFORZIA® for up to ~3.5...
Majority of patients experienced low rates of adverse events which declined in frequency and severity with continued treatment.
Study Shows Negative Impact of Avoidance and Fear of Accidental Reactions on Allergic Kids...
The psychosocial impact of food allergies on children and teens is severe.
Results of Aimmune’s Pivotal Phase 3 European Trial of PALFORZIA® Published in The Lancet...
Peanut-allergic patients treated with Palforzia showed desensitization to peanut protein with a predictable safety profile at nine months
New Two-Year PALFORZIA™ Data Show On-going Safety and Efficacy and Continued Immunomodulation in Patients...
After 2 Years of Daily Treatment, More Than 80% of Patients Were Successfully Desensitized to 2000 mg Peanut Protein or Equivalent of About 14 Peanut Kernels.
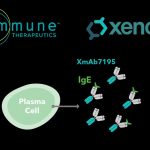
Aimmune Licenses Exclusive Worldwide Rights to Xencor’s XmAb®7195 for the Development of Next-Generation Food...
“In-licensing AIMab7195 demonstrates our commitment to enriching our pipeline and strengthening Aimmune’s global leadership in the evolving therapeutic landscape of food allergy treatments.”