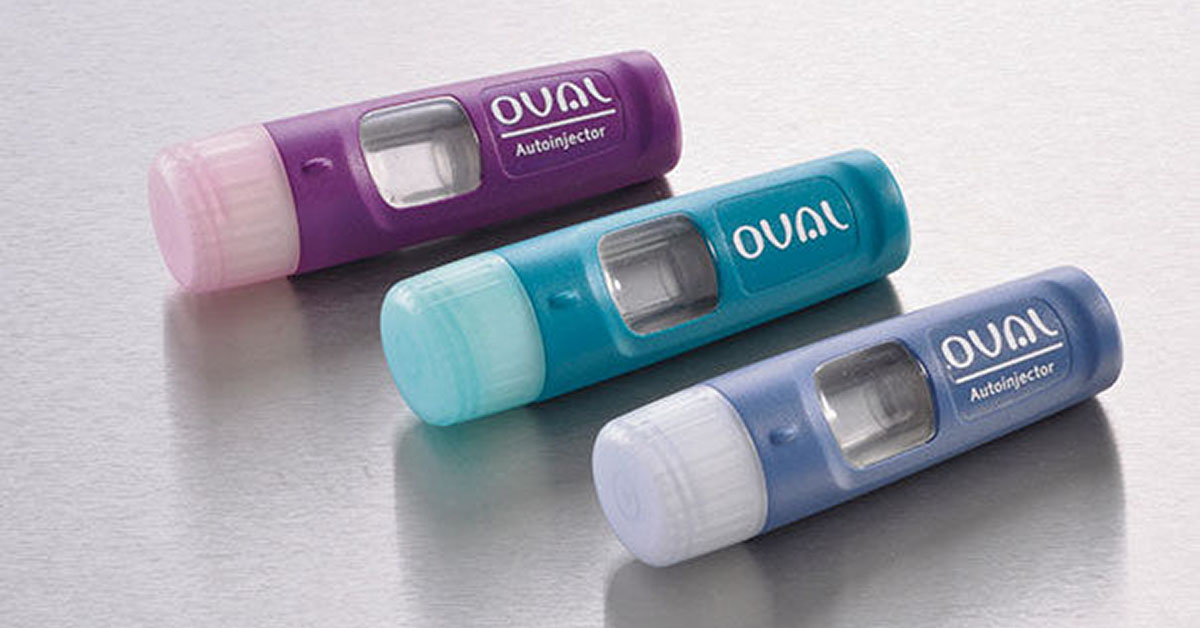
Oval Medical Technologies – a Cambridge, UK based medical device company founded in 2009 – describes its key aims as follows:
- To design an autoinjector that is safe and easy to use in all patient populations.
- To provide a less contaminating environment for the storage of all drugs including fragile biologics
- To significantly improve the quality and reliability of autoinjectors
The firm is currently in the early stages of testing the design of a new, compact auto-injector which the company estimates may hit the UK market in 2019.
In an independent study commissioned by the firm, 21 allergy sufferers used the device correctly without receiving prior instruction in the operation of the device. Of the participants, 17 said the compact size was a major advantage while 19 preferred the device to three others currently available.
In a companion study, all 10 participating medical professionals preferred the device to current auto-injectors and believed their patients would be more likely to carry them.
As our readers well know, many reported deaths due to anaphylaxis occur in individuals who do not have their auto-injectors on-hand. Often tragedies, like that of Dylan Hill, occur because traditional auto-injectors are perceived as large, cumbersome and hard to carry and are often left home, especially by teens.
The Oval device as tested is 93mm x 29.75mm x 16mm (3.7in x 1.2in x 0.6in), significantly smaller than current offerings.
Says Barbara Lead, CEO of Oval:
“Patients don’t always carry auto-injectors because they’re too big, and they often don’t know how to use them should an emergency occur – parents too.
“Teenagers often won’t carry them at all – but they are more likely to carry a compact device that fits in their pocket. As our report makes clear, size and usability are two crucial factors in persuading patients to carry auto-injectors and ensuring they’re well prepared for an emergency.
“This market research confirms that Oval’s approach to auto-injector design and our progress to date are addressing a vital global healthcare need affecting young people especially. We are now focused on addressing regulatory trials followed by a product launch in about 2019.”
We will follow the progress of the Oval device and reporting back as news develops. This announcement comes on the heels of the article we published in December covering Windgap Medical’s issuance of a patent for their novel auto-injector design.
We at SnackSafely.com remain cautiously optimistic that consumers will have additional epinephrine auto-injector options to choose from in the coming years, especially important in the wake of Sanofi’s recall of all Auvi-Q and Allerject auto-injectors.
- Company Overview – Oval Medical Technologies
- Oval Medical anti-allergy device wins acclaim – BusinessWeekly