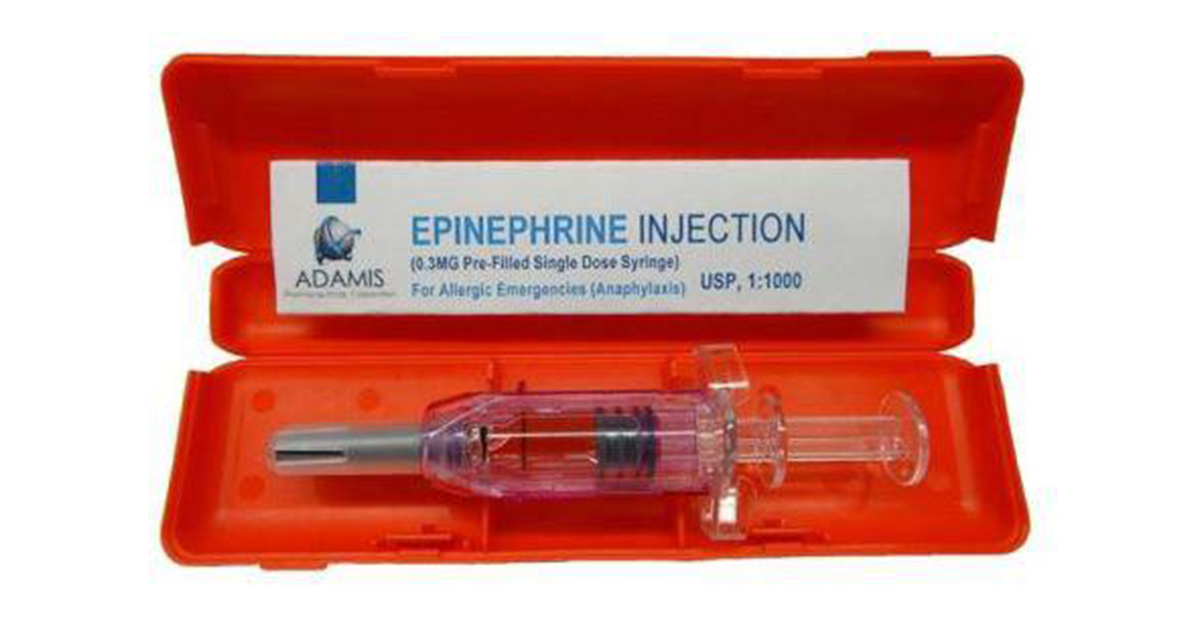
Last month, Adamis Pharmaceuticals Corporation received a Complete Response Letter (CRL) from the US Food and Drug Administration (FDA) sending their Prefilled Single Dose Syringe (PFS) application back for more study. The PFS is intended to provide a lower cost alternative to current epinephrine auto-injectors on the market in the US and abroad.
The rejection is the second for the product which previously received a CRL from the FDA in March of 2015 requiring changes to the product. This time, the FDA is requesting more complete human factors (patient usability) and reliability studies in light of the changes the company made to the product in response to the prior CRL.
The company expects to submit the additional data sometime in the second half of 2016.
At first glance, the device seems less suited to be administered by oneself and better suited for use by experienced responders such as EMTs, firefighters, and police on those they suspect are suffering an anaphylactic episode.