As we reported this past October, BlueWillow Biologics®, a clinical-stage biopharmaceutical company, announced it had been awarded a Fast-Track Small Business Research Innovation (SBIR) contract from the National Institute of Allergy and Infectious Diseases (NIAID) for the development of an intranasal therapeutic peanut allergy vaccine using the company’s NanoVax® platform. The contract provides funding of up to $3.2 million to enable the company to complete preclinical research and prepare to file an Investigational New Drug (IND) application for its candidate vaccine.
Since the awarding of the contract, preclinical studies of the peanut vaccine have been completed while the company has begun preclinical studies of a milk allergy vaccine and is ready to begin those of an egg allergy vaccine.
In animal studies conducted by researchers at the University of Michigan and published in 2018 in The Journal of Allergy and Clinical Immunology, the NanoVax peanut vaccine was effective in treating established allergic disease by suppressing inflammatory allergic responses.
Said BlueWillow CEO Dave Peralta:
Food allergy is a very significant healthcare concern today, and even more alarming is the fact that incidence is increasing. The immunotherapy regimens being studied provide some hope for families with loved ones suffering from food allergies, but these therapies come with risks and limitations. BlueWillow’s peanut allergy vaccine is a unique approach which combines minuscule amounts of purified peanut protein with our novel intranasal NanoVax system and is designed to reprogram the immune system to induce long-term suppression of allergic reactions. Our peanut allergy vaccine has demonstrated this profile in numerous animal studies, and we are thrilled to win this Fast-Track contract which will allow us to advance the program towards Phase I clinical studies.
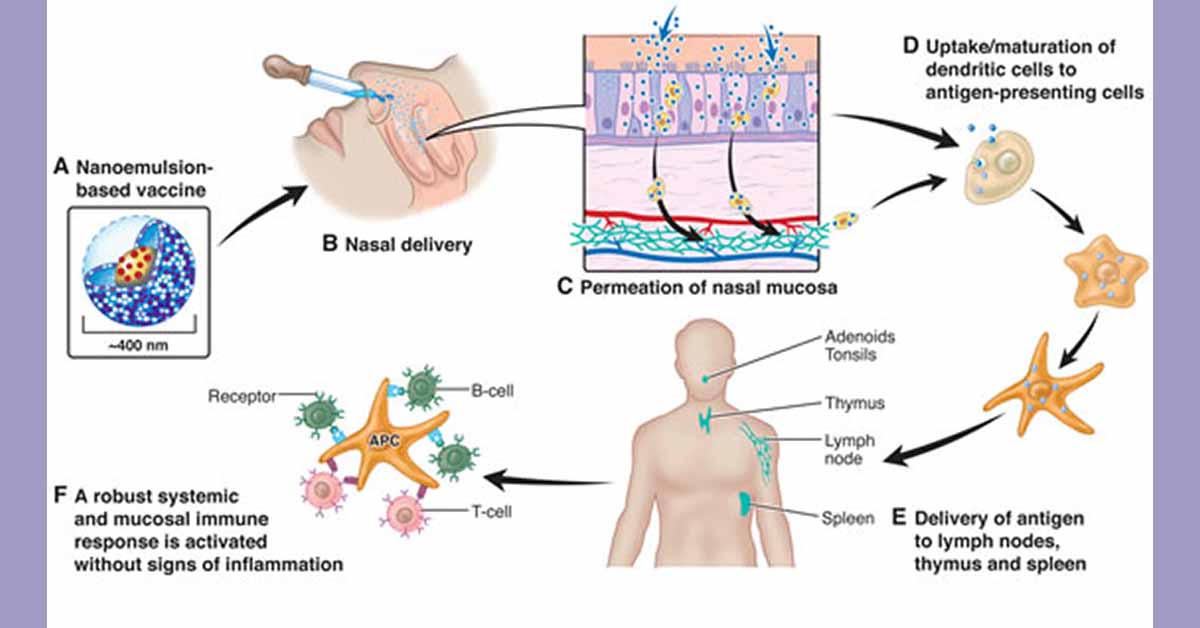
BlueWillow is a clinical-stage, privately-held biopharmaceutical company focused on developing and commercializing prophylactic and therapeutic vaccines using its patented NanoVax platform. The platform employs a novel oil-in-water nanoemulsion adjuvant that is effective when administered intranasally, intramuscularly and topically, and can elicit both mucosal and systemic immunity.
- Our Proprietary Technology Platform — BlueWillow Biologics
- Vaccine Pipeline — BlueWillow Biologics
- BlueWillow Awarded NIH Contract to Advance Development of its Therapeutic Peanut Allergy Vaccine — BlueWillow Biologics
- Nanoemulsion adjuvant–driven redirection of TH2 immunity inhibits allergic reactions in murine models of peanut allergy — JACI
Help Finding Allergy-Friendly Foods: #SnackSafelyAtHome