BRYN PHARMA ANNOUNCES STUDIES EVALUATING EFFECTS OF NASAL CONGESTION ON EPINEPHRINE PHARMACOKINETICS AFTER ADMINISTRATION OF INTRANASAL EPINEPHRINE
Findings Published Online in Respiratory Research
Raleigh, NC – April 8, 2020 – Bryn Pharma, LLC (“Bryn” or the “Company”), a privately held pharmaceutical company dedicated to finding a better way for patients and caregivers to treat anaphylaxis, today announced results from its preclinical studies evaluating intranasal (IN) epinephrine pharmacokinetics and heart rate in a histamine-induced nasal congestion canine model. The results demonstrated that IN epinephrine led to faster epinephrine absorption in the context of histamine-induced nasal congestion, a symptom commonly reported during anaphylactic episodes. Results from the studies were published in Respiratory Research online on April 3, 2020.
Epinephrine is considered to be an effective therapeutic option for the treatment of severe allergy and anaphylaxis. While auto-injectors are effective in reducing anaphylactic symptoms, there can be challenges associated with their route of administration including injuries, fear of needles and administration anxiety resulting in delayed treatment. These challenges point to the need for alternative routes of administration for the delivery of epinephrine.
“Since the release of histamine during an anaphylactic event contributes to vasodilation and congestion, we are pleased that the findings from this preclinical study demonstrated that nasal congestion enhances the absorption of intranasal epinephrine,” said David Dworaczyk, PhD, CEO of Bryn Pharma. “A treatment method like intranasal delivery that is easier and more convenient may lead to increased compliance and result in a reduced time to treat an anaphylactic event.”
Key Study Findings
Pharmacokinetics
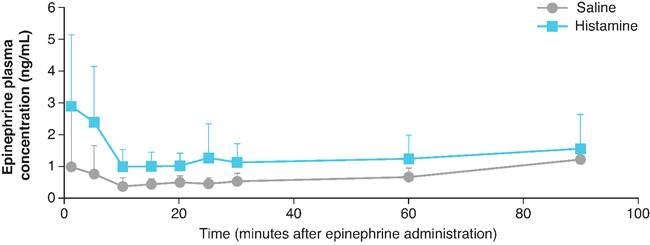
- IN epinephrine led to a greater peak epinephrine concentration (Cmax) in the histamine nasal congestion group versus the saline group (3.5 ng/mL vs 1.7 ng/mL; p = 0.06)
- Significantly shorter time to reach the maximum concentration (Tmax) occurred in the histamine versus saline group after IN epinephrine (6 vs 70 minutes; p = 0.02)
- After IN epinephrine administration, the area under the plasma concentration-time curve from 1 to 90 minutes (AUC1–90)was greater in dogs that received histamine versus saline (117 vs 59 ng/mL*minutes, respectively; p = 0.09)
Effect of IN Epinephrine on Epinephrine Plasma Concentrations After IN Histamine or Saline.
Heart Rate
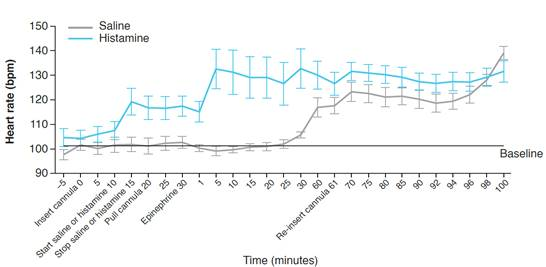
- After IN epinephrine, the histamine versus saline groups showed immediate (5 minutes) versus delayed (30–60 minutes) increases in heart rate, although heart rate
was slightly elevated at the time of epinephrine administration in dogs receiving histamine
Effect of IN Epinephrine on Heart Rate After IN Histamine or Saline
About the Clinical Development Program for BRYN-NDS1C
Bryn’s Bi-Dose Epinephrine Nasal Spray (BRYN-NDS1C) is a single, portable, needle-free device capable of delivering two therapeutic doses of epinephrine, replacing the need to carry two epinephrine auto-injectors. In early 2019, the U.S. Food and Drug Administration (FDA) granted Fast Track Designation to BRYN-NDS1C. In October 2019 the Company completed dosing in the pivotal human trial designed to support U.S. approval to market the product candidate. BRYN-NDS1C is not currently approved for sale by the FDA or any international regulatory authority.
About Anaphylaxis
Anaphylaxis is a serious, life-threatening allergic reaction. The most common anaphylactic reactions are to foods, insect stings, medication and latex.1 A major difference between anaphylaxis and other allergic reactions is that anaphylaxis typically involves more than one system of the body.2 Anaphylaxis requires immediate medical treatment, driving approximately 100,000 emergency room visits in the U.S. each year.1,3 Because 30% of patients who develop anaphylaxis will require a second dose of epinephrine to control symptoms, practice parameters recommend that physicians provide patients with two auto-injectors.4 If not treated properly, anaphylaxis can be fatal.2 However, studies have shown that the majority of people at risk for anaphylaxis often do not carry two epinephrine auto-injectors due in part to size and cost of the products, putting patients at greater risk of severe complications during an allergic reaction.
About Bryn Pharma
Bryn Pharma, founded in 2016, is a privately held pharmaceutical company founded by patients for patients. Bryn is focused on positively disrupting the existing market for epinephrine auto-injectors by delivering an accessible, easy-to-use alternative that better meets the needs of patients. Bryn Pharma seeks to provide this growing population at risk for anaphylaxis with A Better Way to be prepared for a life-threatening allergic reaction. For more information visit www.brynpharma.com.
# # #
Forward-Looking Statements
Statements made in this press release that look forward in time or that express beliefs, expectations or hopes regarding future occurrences or anticipated outcomes or benefits are forward-looking statements. A number of risks and uncertainties, such as risks related to product development and commercialization efforts, results of clinical trials, ultimate clinical outcomes and benefit of the Company’s products to patients, market and physician acceptance of the Company’s products, intellectual property protection and competitive product offerings, could cause actual events to differ from the expectations indicated in these forward-looking statements. You are cautioned not to put any undue reliance on any forward-looking statement. This press release is neither an offer to sell nor a solicitation of an offer to purchase any particular securities. Any such offer or solicitation will be made only pursuant to definitive legal agreements prepared specifically for such purpose. An investment in the Company’s securities entails significant risks and is suitable only for sophisticated investors who can afford a loss of their entire investment; no assurance can be given that investment objectives will be achieved. In considering the performance information contained herein, you should bear in mind that past performance is not necessarily indicative of future results; there can be no assurance that the Company will achieve comparable results or that any projected returns will be met. The Company does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
- American Academy of Allergy, Asthma & Immunology. Available at https://www.aaaai.org/conditions-and-treatments/allergies/anaphylaxis. Accessed on February 21, 2020.
- American Academy of Allergy, Asthma & Immunology. Available at: https://www.aaaai.org/conditions-and-treatments/conditions-dictionary/anaphylaxis. Accessed on February 21, 2020.
- Fromer L. Prevention of Anaphylaxis: The Role of the Epinephrine Auto-Injector. Am J Med. 2016 Dec; 129(12): 1244-1250.
- Anaphylaxis – a practice parameter update 2015; Lieberman, Phillip et al.; Annals of Allergy, Asthma & Immunology, Volume 115, Issue 5, 341-384.