Home Search
dbv - search results
If you're not happy with the results, please do another search
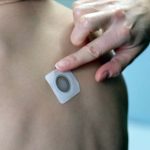
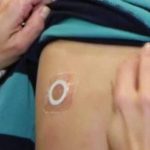
FDA Denies Approval of Viaskin Peanut “Patch” Therapy
The FDA raised concerns that adhesion of the patch would affect the efficacy of the therapy.
Will the Viaskin “Patch” Therapy for Peanut Allergy be Approved Soon? The Company is...
Company is restructuring in anticipation of delays.
FDA Advisory Committee Meeting to Review Viaskin Peanut for the Treatment of Peanut Allergy...
Next step in the approval process for the therapy scheduled for May 15, 2020.
FAQ for Palforzia, the First FDA Approved Therapy for Food Allergy
What it is, what it treats, who it is for, how it is used, et al.
SnackSafely.com Newsletter: 2019/08/08
DBV Technologies Submits Biologics License Application to FDA for Viaskin Peanut for the Treatment of Peanut Allergy (+4 Articles)
Fare Responds to Final ICER Review of Breakthrough Peanut Allergy Therapies
FARE expresses its deep concerns with the final evidence report on two breakthrough peanut allergy therapies issued Wednesday by ICER.
Asthma and Allergy Foundation of America Responds to Premature ICER Review of New Peanut...
The AAFA addresses concerns
regarding the Final Evidence Report released by the ICER.
ICER’s Final Report on Peanut Allergy Treatments: Clinical Evidence Does Not Yet Demonstrate That...
The report evaluates Viaskin® Peanut and AR101, as well as non-commercialized oral immunotherapy (OIT).
Asthma and Allergy Foundation of America Elevates Patient Voice in ICER Review of New...
Affirms Burden of Food Allergies on Families, Patients, and Caregivers.
Joint Statement on Peanut Allergy Therapy Review
Organizations representing patients and allergists urge ICER to adopt a more patient-driven approach to assessing immunotherapies for peanut allergy.