Home Search
dbv - search results
If you're not happy with the results, please do another search
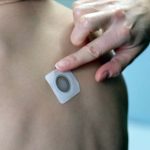
Viaskin Peanut Completes Phase III Part A Study of Toddlers With No Safety Concerns
Part B expected to commence in 2018Q4 with Viaskin Peanut 250 µg.
Milk and Peanut Clinical Trial Advances
Announcements regarding two food allergy therapies that are in or advancing toward clinical trials were made yesterday.
Positive Safety Results Reported for Viaskin Peanut Phase III Trial
The trial met its primary objective, demonstrating that Viaskin Peanut was well-tolerated with no new or unexpected adverse events.
Viaskin Peanut Phase III Trial Falls Short, Still Chance for FDA Approval
The Viaskin Peanut patch therapy still has 50:50 chance of approval according to research note.
Peanut Patch Phase III Enrollment Ends, Results Expected Toward End of 2017
The study received higher-than-expected patient demand.
Study: Peanut Immunotherapy Significantly Reduces Risks Due to Cross-Contact in Common Packaged Foods
The study model used common packaged foods such as cookies, ice cream, and doughnuts as references.
Viaskin Peanut Patch: Lasting Response After Three Years
Pediatric patients responded with a favorable safety profile and no serious adverse effects.
Viaskin Patch Delivers Long-Lasting Peanut Desensitization After Phase IIb Trial
The study was designed to assess the long-term efficacy and safety of the treatment in subjects that had graduated from their earlier VIPES trial.
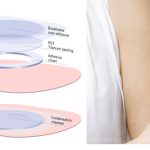
Study: EPIT (Skin Patch) Therapy Provides Sustained Protection Against Anaphylaxis
The unique immune communication between skin and gastrointestinal tract can be used to generate long-lasting protection from food allergies.
Viaskin Peanut, Milk and Egg Patch Therapies: Promising News from the AAAAI Conference
We are cautiously optimistic that a safe, effective, easy to administer treatment for food allergy may be on the horizon.