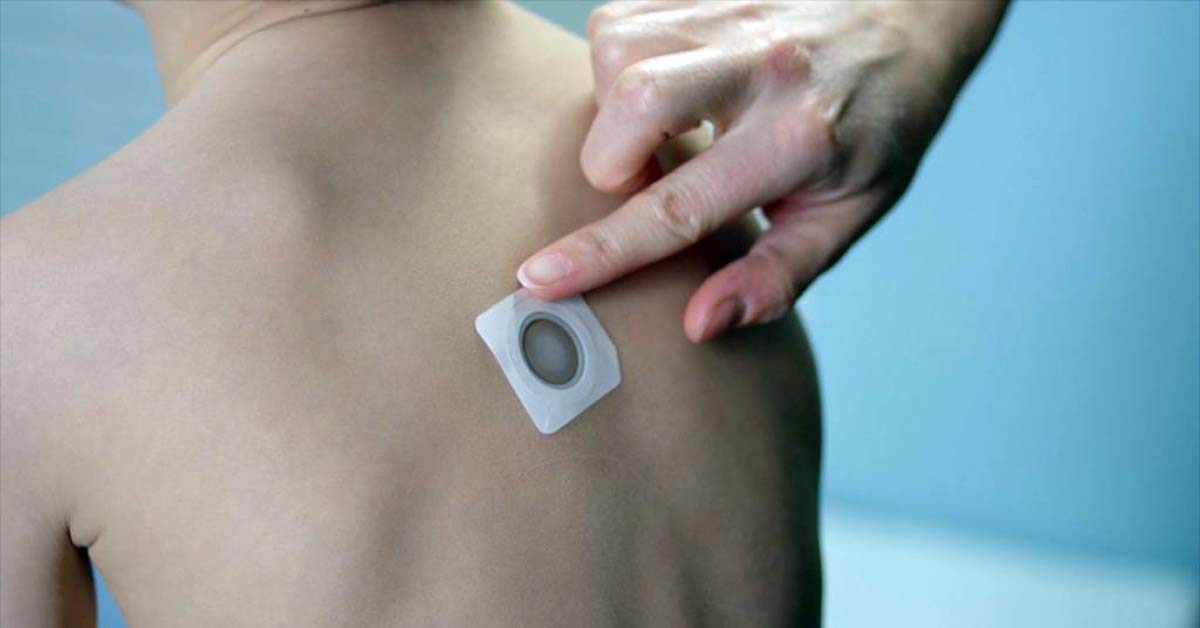
As our readers are well aware, DBV Technologies — a pharmaceutical company headquartered in Paris, France — is developing a family of epicutaneous (on the skin) desensitization therapy for a number of food allergens. The Viaskin “patches” are worn on the skin, exposing the patient to low doses of an allergen over time, thus training the immune system to tolerate successively higher doses of inadvertent exposure to the allergen. DBV’s first candidate is a peanut therapy with therapies for milk and egg in the pipeline.
Back in March, DBV received feedback from the US Food and Drug Administration (FDA) that it had questions regarding the impact of patch adhesion on the efficacy of the therapy, threatening to push back approval and issuance of a Biologic License to begin offering the therapy.
In a recent press release, the company explained how they are proceeding given the uncertainty surrounding the approval of the therapy, which may be pushed back further from the current target date in August.
Said Daniel Tassé, Chief Executive Officer of DBV Technologies:
We have carefully reviewed the situation and given the prevailing uncertainties,
the goal of the plan that we are launching aims to preserve our core functions,
extend our cash runway and maintain operating latitude to bring the first and only
epicutaneous immunotherapy for the treatment of peanut allergy to patients in
need, if approved.We continue to believe Viaskin Peanut, based on the clinical data observed to
date, can change the long-term treatment paradigm of peanut allergies and lend
itself well to this current healthcare environment.
The press release goes on to say the company will divert resources from their other (pre-)clinical programs to focus on pushing Viaskin peanut across the finish line, meaning their therapies for milk and egg allergies will be delayed in addition to the delays their peanut therapy is facing.
DBV’s Viaskin Peanut application with the FDA focuses on children ages 4-11 years. The press release provides an encouraging update on their ongoing EPITOPE trial, a two-part,
pivotal Phase III clinical trial assessing the safety and efficacy of Viaskin Peanut for
the treatment of peanut-allergic toddlers ages 1 to 3 years.
Said Pharis Mohideen, M.D., Chief Medical Officer of DBV Technologies:
These initial data from EPITOPE, our second Phase III clinical trial for Viaskin
Peanut, provide support for an epicutaneous immunotherapy approach to
treating food allergy in this age group. There is a growing body of scientific evidence that
supports the importance of treating peanut-allergic patients from a young age,
when they are most likely to be diagnosed. These findings are important not only
for understanding the science of epicutaneous immunotherapy in different age
groups, but also for advancing a potential treatment option for very young
patients.