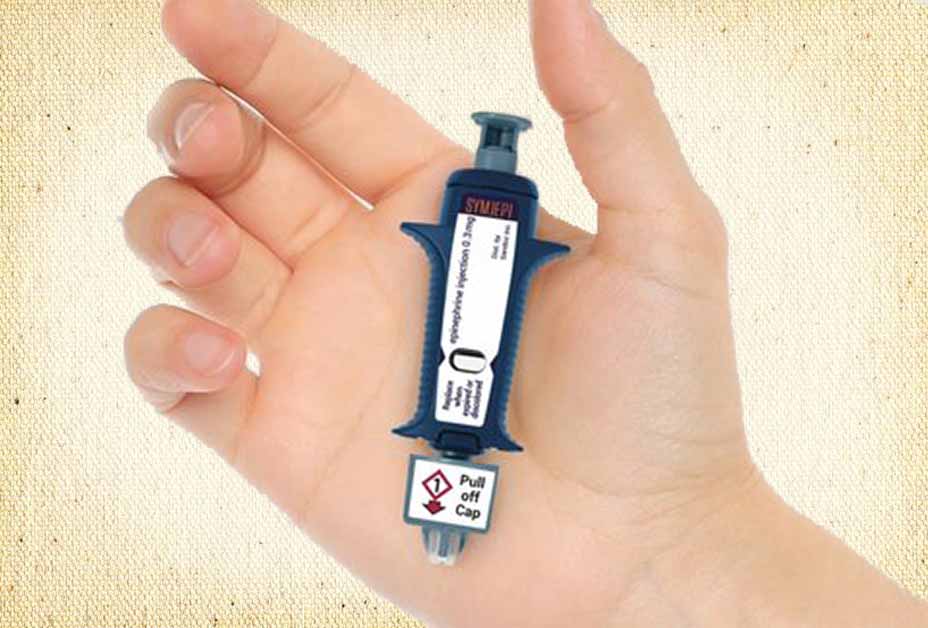
PRINCETON, N.J., Jan. 16, 2019 /PRNewswire/ — Sandoz Inc. (Sandoz), a Novartis division, today announced the US market introduction of SYMJEPI™ (epinephrine) 0.3 mg Injection for the emergency treatment of allergic reactions (Type 1), including anaphylaxis. Sandoz is launching this medicine as an affordable, single-dose, pre-filled syringe alternative to epinephrine auto-injectors.
SYMJEPI will be rolled out through a phased launch, first in the institutional setting – an established channel where Sandoz Inc. has significant experience and knowledge – followed by introduction into the retail market.
SYMJEPI 0.3 mg Injection is indicated for the emergency treatment of allergic reactions (Type 1) including anaphylaxis to stinging and biting insects, allergen immunotherapy, foods, drugs, diagnostic testing substances and other allergens, as well as idiopathic or exercise-induced anaphylaxis. SYMJEPI 0.3 mg Injection is intended for immediate administration in patients who weigh 30 kg (approximately 66 pounds) or more, and who are determined to be at an increased risk for anaphylaxis, including individuals with a history of anaphylactic reactions.
“The SYMJEPI device is small in size and fits into the palm of your hand, with the goal of a simple-to-use application and intuitive, user-friendly design,” said Carol Lynch, President of Sandoz Inc. “At Sandoz, we strive to reimagine medicine to offer the best care we can in all we do, and having heard from patients, caregivers and healthcare professionals about their eagerness for a new product, we are proud to be a part of the solution to a critical need in the US.”
Important Safety Information
CONTRAINDICATIONS
None
WARNINGS AND PRECAUTIONS
- You should get emergency medical care right away after using the product.
- You may need to use a second SYMJEPI (epinephrine) injection if symptoms continue or recur. Only a healthcare provider should give additional doses of epinephrine if you need more than 2 injections for a single anaphylaxis episode.
- SYMJEPI should only be injected into the middle of your outer thigh (upper leg) with the needle facing downwards. Never inject into any other part of the body. If you accidentally inject SYMJEPI into any other part of your body, go to the nearest emergency room right away. Tell the healthcare provider where on your body you received the accidental injection. SYMJEPI can be injected through your clothing if needed.
- The needle cap on the SYMJEPI prefilled syringe helps to prevent needle sticks and accidental injection of epinephrine. Do not remove the needle cap until you are ready to use it.
- Never put your thumb, fingers, or hand over the exposed needle.
- If an accidental injection happens, get medical help right away.
- Do not drop the carrier case or SYMJEPI prefilled syringe. If the carrier case or prefilled syringe is dropped, check for damage and leakage. Dispose of the prefilled syringe and carrier case, and replace if damage or leakage is noticed or suspected.
- Do not place patient information or any other foreign objects in the carrier case with the prefilled syringe, as this may prevent you from removing the prefilled syringe for use.
- If you inject a young child with SYMJEPI, hold their leg firmly in place before and during the injection to prevent injuries. Ask your healthcare provider to show you how to properly hold the leg of a young child during injection.
- Before using SYMJEPI, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems or high blood pressure.
- have diabetes.
- have thyroid problems.
- have asthma.
- have a history of depression.
- have Parkinson’s disease.
- are pregnant or plan to become pregnant. It is not known if epinephrine will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if epinephrine passes into your breast milk.
ADVERSE REACTIONS
SYMJEPI may cause serious side effects.
- Rarely, patients who have used SYMJEPI may develop infections at the injection site within a few days of an injection. Some of these infections can be serious. Call your healthcare provider right away if you have any of the following at an injection site:
- redness that does not go away
- swelling
- tenderness
- the area feels warm to the touch
- Cuts on the skin, bent needles, and needles that remain in the skin after the injection, have happened in young children who do not cooperate and kick or move during the injection.
- If you have certain medical conditions, or take certain medicines, your condition may get worse or you may have longer lasting side effects when you use your SYMJEPI. Talk to your healthcare provider about all your medical conditions.
Common side effects of SYMJEPI include:
- fast, irregular or “pounding” heartbeat
- sweating
- headache
- weakness
- shakiness
- paleness
- feelings of over excitement, nervousness or anxiety
- dizziness
- nausea and vomiting
- breathing problems
These side effects may go away with rest. Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of SYMJEPI. For more information, ask your healthcare provider or pharmacist.
DRUG INTERACTIONS
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
- Tell your healthcare provider of all known allergies.
- Especially tell your healthcare provider if you take certain asthma medicines.
- SYMJEPI and other medicines may affect each other, causing side effects. SYMJEPI may affect the way other medicines work, and other medicines may affect how SYMJEPI works.
- Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
See full PI for additional safety information available at https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b1c6a201-4f23-489f-9fca-f8c82bb9fa58
You are encouraged to report negative side effects of prescription drugs to FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Disclaimer
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements can generally be identified by words such as “potential,” “can,” “will,” “plan,” “expect,” “anticipate,” “look forward,” “believe,” “committed,” “investigational,” “pipeline,” “launch,” or similar terms, or by express or implied discussions regarding potential marketing approvals, phased launches, new indications, or labeling for SYMJEPI, or regarding potential future revenues from SYMJEPI. You should not place undue reliance on these statements. Such forward-looking statements are based on our current beliefs and expectations regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that SYMJEPI will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Neither can there be any guarantee that SYMJEPI will be successfully launched, or at any particular time. Nor can there be any guarantee that SYMJEPI will be commercially successful in the future. In particular, our expectations regarding SYMJEPI could be affected by, among other things, the uncertainties inherent in research and development, including clinical trial results and additional analysis of existing clinical data; regulatory actions or delays or government regulation generally; the particular prescribing preferences of physicians and patients; competition in general, including potential approval of additional competitive versions of this product; global trends toward health care cost containment, including government, payor and general public pricing and reimbursement pressures; litigation outcomes, including intellectual property disputes or other legal efforts to prevent or limit Sandoz from selling its products; general political and economic conditions; safety, quality or manufacturing issues; potential or actual data security and data privacy breaches, or disruptions of our information technology systems, and other risks and factors referred to in Novartis AG’s current Form 20-F on file with the US Securities and Exchange Commission. Novartis is providing the information in this press release as of this date and does not undertake any obligation to update any forward-looking statements contained in this press release as a result of new information, future events or otherwise.
About Sandoz
Sandoz is a global leader in generic pharmaceuticals and biosimilars. As a division of the Novartis Group, our purpose is to discover new ways to improve and extend people’s lives. We contribute to society’s ability to support growing healthcare needs by pioneering novel approaches to help people around the world access high-quality medicine. Our portfolio of approximately 1000 molecules, covering all major therapeutic areas, accounted for 2017 sales of USD 10.1 billion. In 2017, our products reached well over 500 million patients. Sandoz is headquartered in Holzkirchen, in Germany’s Greater Munich area.