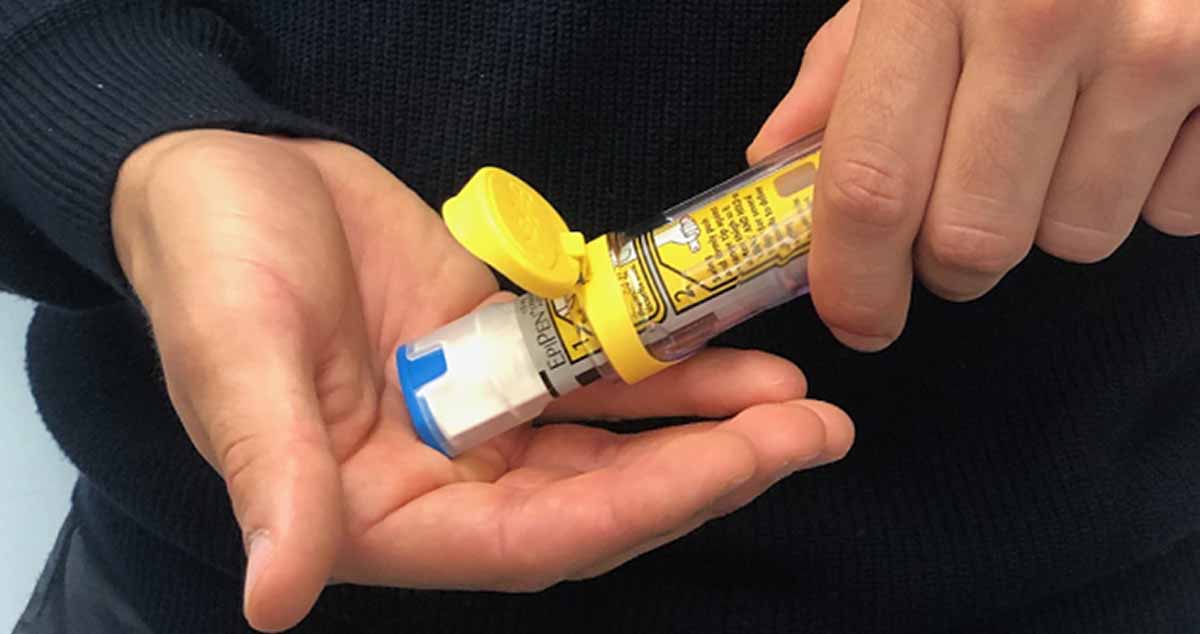
Consumers and pharmacists should check that devices can be removed from their carrier tubes with ease
October 11, 2019
For immediate release
OTTAWA – Pfizer Canada has advised Health Canada that, in a very small number of cases, some EpiPen (0.3 mg) and EpiPen Jr (0.15 mg) auto-injector devices may not slide out of their carrier tube easily, or at all. Although the chance of this occurring is very rare, failure to administer epinephrine as soon as possible during an anaphylactic response could lead to patient disability or death. The number of EpiPen or EpiPen Jr auto-injectors with this issue is estimated to be two auto-injectors out of every one million.
According to the company, a very small number of carrier tubes have a defect that partially blocks the opening of the tube and may prevent easy release of the auto-injector from the carrier. The issue is with the carrier tube, and not with the device itself or the drug that it delivers (epinephrine). The company has identified the root cause of this issue and put corrective measures in place.
Products are not being recalled by Pfizer because the risk can be easily addressed by consumers and pharmacists checking devices—before an emergency situation arises—to make sure they slide easily out of their carrier tube. Should you have a device with this defect, please return it to your pharmacist for replacement.
EpiPen and EpiPen Jr are used to deliver an emergency treatment of adrenaline (epinephrine) to patients who are at risk or have a history of life-threatening allergic reactions (anaphylaxis).
According to the company, there have been no reports of malfunctions related to this specific issue in Canada.
Who is affected
- Patients and caregivers who have the affected product
Affected products
EpiPen (0.3 mg) (DIN 00509558) and EpiPen Jr (0.15 mg) (DIN 00578657) products expiring on or before September 2020
What consumers should do
Pfizer Canada is advising consumers to check whether their device expires on or before September 2020, and if so, to do the following (images are provided below):
- Check your device to make sure that it slides out easily from the carrier tube. To check the device, flip open the carrier tube cap, gently turn the tube upside down and let the device slide out into your hand (do not shake or drop it). Visually inspect the carrier tube to make sure that the open rim of the carrier tube is not deformed.
- Do NOT remove the blue safety release from the auto-injector device. The blue safety release should be kept on the auto-injector until the time of use.
- Return the EpiPen device to its carrier tube and close the carrier tube flip cap once you have confirmed that your device slides out easily and that there are no deformities on the open rim of the carrier tube.
- If your device does not slide out easily from its tube, or the open rim of the plastic carrier tube appears deformed, return it to your pharmacist for replacement.
- If you are unsure about how to check your device, your pharmacist can check your device for you.
In addition, Health Canada recommends that you:
- Develop a habit of checking a new auto-injector when it is received to ensure the device can be easily removed from its packaging. Although problems with removal are rare, they have occurred on previous occasions and may reoccur periodically.
- Report adverse events involving health products to Health Canada by calling toll-free at 1‑866‑234‑2345, or by reporting online, by mail or by fax.
- Report complaints about health products to Health Canada by calling toll-free at 1‑800‑267‑9675, or complete an online complaint form.
Additional information for pharmacists and other health professionals:
Additional information for pharmacists and other healthcare professionals has been communicated by Pfizer Canada.
What Health Canada is doing
Health Canada is monitoring this issue and the company’s implementation of corrective measures to prevent this issue from reoccurring. Should additional safety issues be identified with these products, Health Canada will take appropriate action and inform Canadians as necessary.
Background
EpiPen and EpiPen Jr epinephrine auto-injectors are supplied in a carrier tube. The auto-injector is removed from the carrier tube before use by flipping open the cap and inverting the carrier tube to allow the auto-injector to slide out. In a very small number of cases, there is a possibility that the auto-injector may not easily slide out of the tube. This may slow or prevent the administration of epinephrine during an anaphylactic reaction. Although the chance of this occurring as a result of this issue is very rare, failure to administer epinephrine as soon as possible during an anaphylactic response could lead to patient disability or death. Harm is preventable by checking the device before it is needed, to make sure that it will be easily accessed during an emergency.
Stay connected with Health Canada and receive the latest advisories and product recalls.
Media Enquiries:
Health Canada
(613) 957-2983
Public Enquiries:
(613) 957-2991
1-866 225-0709
Image Information
Image 1 – Check whether the device expires on or before September 2020.

Image 2 – Flip open the cap of the carrier tube. The carrier tube cap is yellow for EpiPen (0.3 mg) and green for EpiPen Jr (0.15mg).

Image 3 – Make sure the device slides out easily from its carrier tube by gently turning the tube upside down and letting the device slide out into your hand (do not shake or drop it). Visually inspect the carrier tube to make sure that the open rim of the carrier tube is not deformed. Do NOT remove the blue safety release from the auto-injector. The blue safety release should be kept on the auto-injector until the time of use.

Once you have confirmed that the device slides out easily and there are no deformities on the open rim of the carrier tube, return the EpiPen into its carrier tube and close the carrier tube flip cap. The auto-injector may be dispensed and administered.