Home Search
viaskin - search results
If you're not happy with the results, please do another search
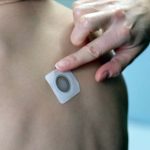
Viaskin Milk Results Shows Potential for Treatment of Milk Allergy in Children
95.5% of the participants completed treatment, with most adverse effects being mild or moderate reactions at the application site.
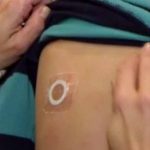
DBV Announces Promising 2-Year Results from Ongoing Viaskin “Peanut Patch” Toddler Study
81.3% of subjects who completed the oral food challenge reached an eliciting dose of ≥1,000 mg after 24 months of treatment.
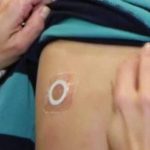
DBV Outlines Path to Regulatory Approval of Viaskin Peanut Patch
Are Viaskin therapies finally on the horizon?
FDA Issues Partial Hold on DBV Viaskin Peanut Patch Trial
Another delay for the long-awaited therapy.
DBV Begins Phase 3 Study of Redesigned Viaskin Peanut Patch in Children Ages 4-7
Viaskin is testing a new version of their patch designed to address the FDA's concerns.
DBV Announces Positive Topline Results from Phase 3 Trial of Viaskin Peanut in Peanut-Allergic...
Viaskin Peanut demonstrated a statistically significant treatment effect with 67.0% of subjects meeting the treatment responder criteria after 12 months.
Viaskin Peanut Patch Therapy Delayed Further as DBV Readies Another Phase 3 Trial
The patch therapy remains in regulatory limbo.
Safety of Viaskin Peanut Patch Therapy Improved Over 3-Year Study
Reactions decreased over study duration.
FDA Wants More Viaskin Peanut Data Resulting in Further Approval Delays
Delays mount pushing trials of modified patch.
DBV Optimistic Viaskin Peanut “Patch” Therapy has Path to Approval After FDA Feedback
DBV, which also has Viaskin therapies for milk and egg allergies in their pipeline, is encouraged by FDA feedback after denial of their peanut therapy.