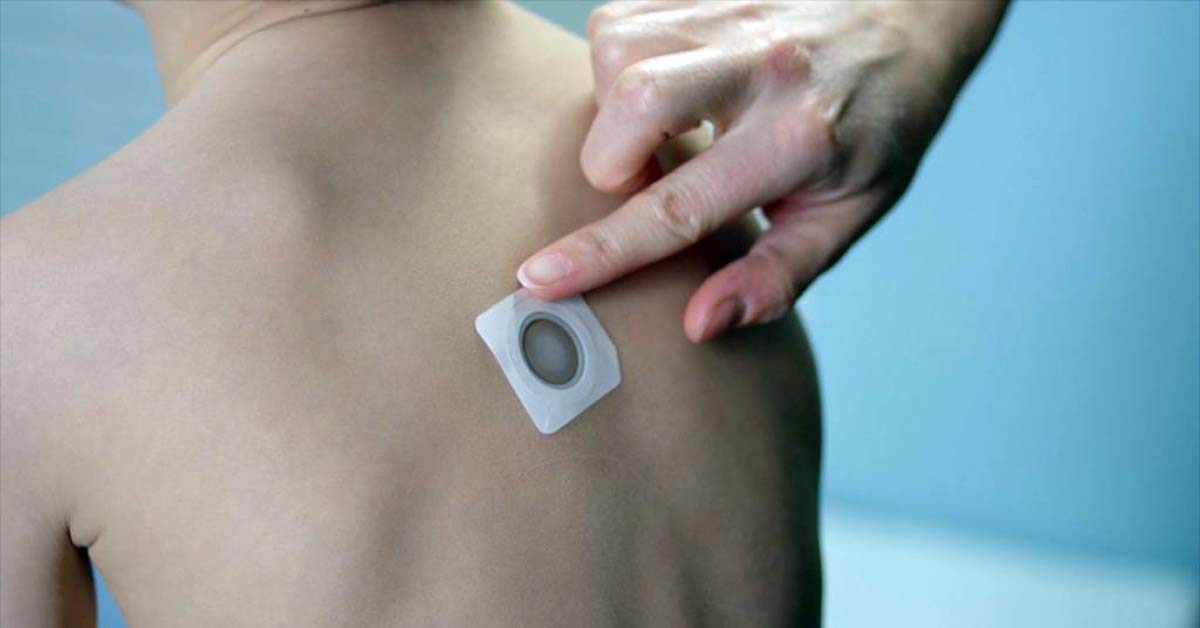
DBV Technologies’ Viaskin Peanut epicutaneous immunotherapy, which consists of a series of patches worn on the back to desensitize the peanut-allergic patient to reactions from inadvertent peanut ingestion, was submitted to the US Food and Drug Administration (FDA) for approval in October of 2019. It remains in regulatory limbo.
The FDA warned DBV in March the following year that it had concerns about the ability of the patch to remain adhered to the skin. The company then designed a new patch that is 50% larger and has been working on a strategy with the FDA to gain approval.
On Monday, the company announced that it had decided to move forward with a new phase 3 clinical study rather than go through a sequential approach of at least five rounds of exchanges with the FDA. The FDA approves of DBV’s new approach.
The company will submit their study protocol to the FDA in February. It is unclear how long it will take the FDA to consider the protocol, for the study run, for the company to write up and submit results to the FDA, and for the FDA to finally approve the therapy. Suffice it to say, it will be some time before the patch becomes available to the peanut-allergic.
Said Daniel Tasse, DBV CEO:
DBV is confident that a new, Phase 3 pivotal study generating a robust data set is the best way to support the development of Viaskin Peanut. In October, we were surprised to see the FDA request a sequential approach to our development plans. Considering the advice and information requests received by FDA concerning STAMP in October and the allergen uptake/transport study in November, the Company has determined that further exchanges with FDA under resource dependent review timelines are unpredictable and would likely result in extended delays to our regulatory progress. We believe Viaskin Peanut is a viable treatment option for patients that are currently underserved and eagerly awaiting options. It is our priority to bring a safe, efficacious, and convenient product to them as quickly as possible.
The company also announced it will be withdrawing its Marketing Authorization Application accepted by the European Medicines Agency in November 2020, presumably to avoid similar delays to those encountered with the FDA.
Said Pharis Mohideen, Chief Medical Officer, DBV Technologies:
The team has been thoughtful and analytical in designing a new, Phase 3 pivotal trial protocol to support the U.S. and European regulatory pathways and will continue to work closely with the EMA as we generate additional data. There is a significant need for effective and well-tolerated therapies for those living with peanut allergy, and we are committed to bringing Viaskin Peanut to patients and physicians as quickly as possible.
DBV also has Viaskin therapies in their pipeline for milk and egg allergies.