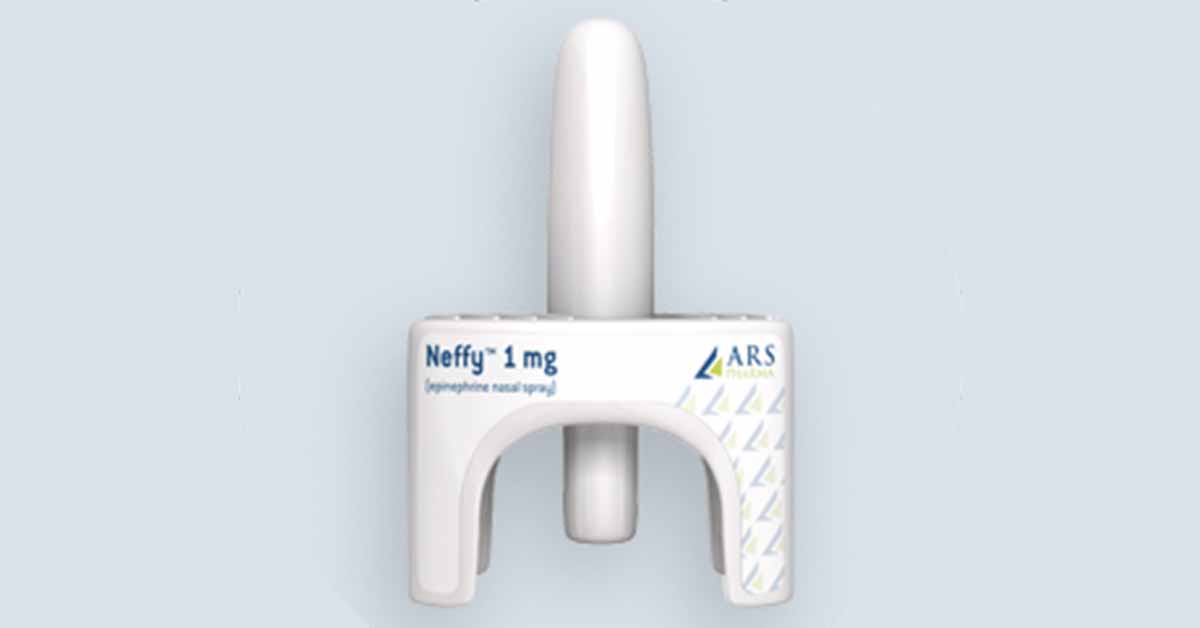
Patent approval covers fast-acting decreased dosage level for intranasal administration for type 1 allergic reaction.
SAN DIEGO (July 13, 2020) – ARS Pharmaceuticals today announced that on June 16, 2020, the United States Patent and Trademark Office issued a second key patent for ARS-1, a low dose intranasal epinephrine nasal spray currently in clinical development. This dosage is significantly lower than other reported investigational intranasal epinephrine projects in development and thus helps protects against possible accidental overdose risk during a severe allergic reaction. This follows a patent approved last year covering the composition of matter of ARS-1. ARS Pharmaceuticals is dedicated to putting patients first and these ARS-1 innovations are an important step for those with severe allergic reactions to get lifesaving, pain free treatment.
Patients need to act quickly when they have a severe allergic reaction. Fear of needles and apprehension to dose autoinjectors can result in a significant delay of epinephrine administration, losing critical time and resulting in poor outcomes and potentially death. A new innovation in nasal technology using Intravail® as an absorption enhancing agent allows ARS-1 (1 mg dose) to achieve bioequivalent exposure with a 0.3 mg epinephrine IM injection or EpiPen, with rapid absorption (time to peak plasma level). The low 1 mg dose, demonstrated to have bioequivalent exposure to a 0.3 mg IM injection, is important to ensure patient safety as other attempts reported in the literature to get nasal absorption are using very high intranasal doses that create the potential for overdose. The combination of Intravail®, the novel drug absorption enhancer in ARS-1 and epinephrine are covered under a previously issued composition of matter patent (U.S. Patent No. 10,576,156) with expiration in 2038..
The new patent issued by the US Patent Office to ARS on June 16, 2020 (U.S. Patent No. 10,682,414), also with expiration in 2038, covers methods related to the use and intranasal administration for a dose range of 0.1 to 2.4 mg epinephrine for the treatment of a type 1 hypersensitivity allergic reaction with any nasal formulation. This newly issued patent is directed at not only Intravail-based epinephrine nasal sprays, but more broadly at any low-dose (0.1 to 2.4 mg) epinephrine nasal spray product containing any absorption enhancer. The fact that measurable absorption of epinephrine by the intranasal route of administration had never been achieved at doses below 2.5 mg in any previous formulation, emphasizes the novelty of ARS-1 in achieving injection like absorption with only a 1 mg dose.
“This new patent is very significant in progressing our efforts to provide caregivers and patients who suffer from severe allergic reactions a more convenient, less threatening and more reliable method to dose epinephrine quickly. This new Track One patent is one of three primary claims in our international patents filed for ARS-1 to support our worldwide partnering efforts,” said Richard Lowenthal, President and Chief Executive Officer of ARS Pharmaceuticals. “Epinephrine autoinjectors can be seen as scary, painful and bulky to carry. ARS-1 is small, delivered by a very reliable device, and achieves injection like absorption without a needle, which removes the fear and apprehension of using a needle. The ability to achieve rapid absorption and bioequivalent exposure with only a single 1 mg dose is critical to the safety and efficacy of the product. Once approved, we anticipate that ARS-1 will be a reliable, easily administered needle-free treatment to address emergency allergic reactions, and the novelty of the ARS-1 technology has been validated by the issuance of these two patents.”
To learn more about ARS-1, please visit https://ars-pharma.com.
About ARS Pharmaceuticals, Inc.
ARS Pharmaceuticals is dedicated to empowering at-risk patients and caregivers to better protect themselves from severe allergic reactions that could lead to anaphylaxis. The Company is developing ARS-1, an investigational intranasal epinephrine spray with a unique absorption technology that could be easy-to-use, needle-free, convenient and more reliable for patients and loved ones at-risk of severe allergic reactions to food, medications and insect bites that could lead to life-threatening anaphylaxis. For more, visit www.ars-pharma.com.
About Intravail®
Intravail®, which is owned by Neurelis, Inc., is a proprietary excipient that enhances mucosal absorption through two mechanisms: (1) paracellular transport, via the transient opening of tight junctions between cells, and (2) transcellular transport, via vesicle carriers. Intravail enables the non-invasive delivery of a broad range of protein, peptide and small molecule drugs, providing unique advantages in bioavailability, safety, convenience, and effectiveness.
Contact: Margaret Long
margaret.long@porternovelli.com
(571) 445-4428