Moonlight Therapeutics, Inc, a biotechnology company developing treatments for food allergies, announced a $1.9 million grant from the National Institute of Allergy and Infectious Diseases (NIAID). The grant will enable the company to complete its pre-clinical activities and submit an Investigational New Drug Application for the peanut allergy treatment with the Food and Drug Administration.
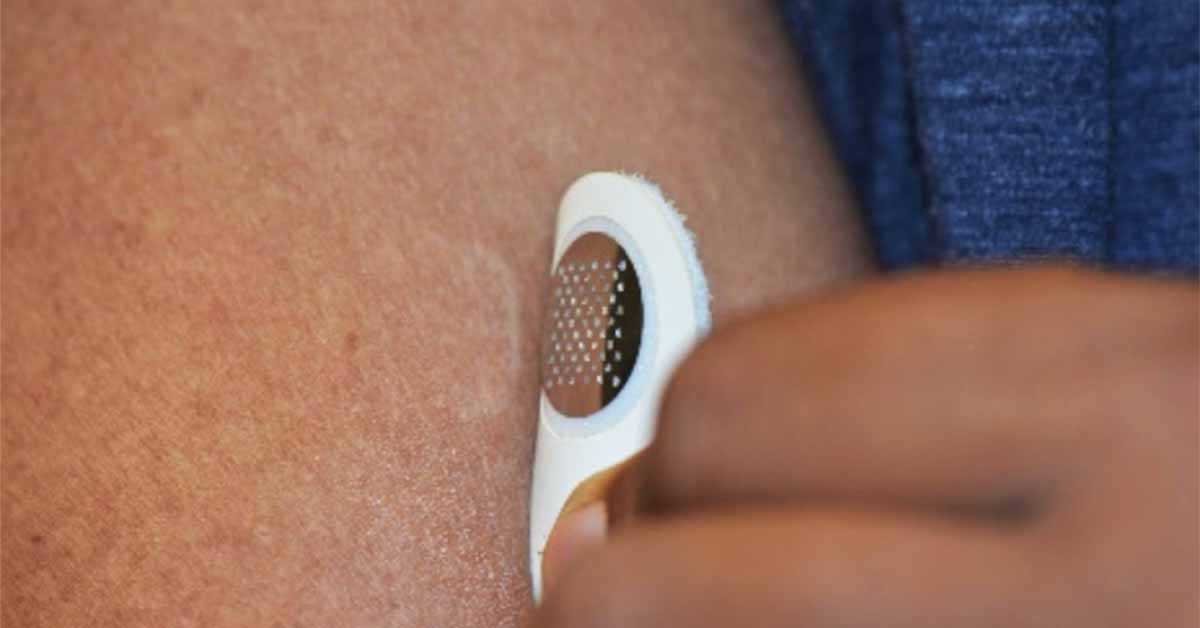
Moonlight’s proprietary treatment platform dubbed TASIS — Targeted Allergen-Specific Immunotherapy within the Skin — involves the application of a small, minimally invasive “dermal stamp” that targets the delivery of allergen proteins to the immune cells in the top layer of skin, subsequently desensitizing the patient from a food allergy over time. The stamp is designed for at-home self-administration and is applied for only a few minutes and then discarded.
The approach has shown encouraging results in animal models of peanut and other allergies.
Said Samir Patel, Moonlight Therapeutics co-founder and CEO:
We are excited to receive the latest funding so that we can move one step closer to bringing this treatment to the clinic. We will now focus on completing our pre-clinical research and begin to raise funding for our first clinical trial in peanut allergic individuals.
Said Dr Brian Vickery, Director of the Food Allergy Center at Emory and Children’s Healthcare of Atlanta:
Allergists have very few treatment options for treating peanut and other food allergies. Moonlight Therapeutics is working on a treatment that has the potential to help address a significant unmet need and we’re eager to evaluate its potential in clinical trials.