Moonlight Therapeutics, a biotechnology company based in Atlanta, announced yesterday a major advance toward the treatment of food allergies, having received clearance from the US Food and Drug Administration (FDA) for its Investigational New Drug (IND) application for MOON101. This clearance allows the company to initiate its first clinical trial in adults and children with peanut allergy.
MOON101 is being developed as a potential new therapy for the over six million people in the United States who live with peanut allergy, a condition for which the unmet need for convenient treatment remains high.
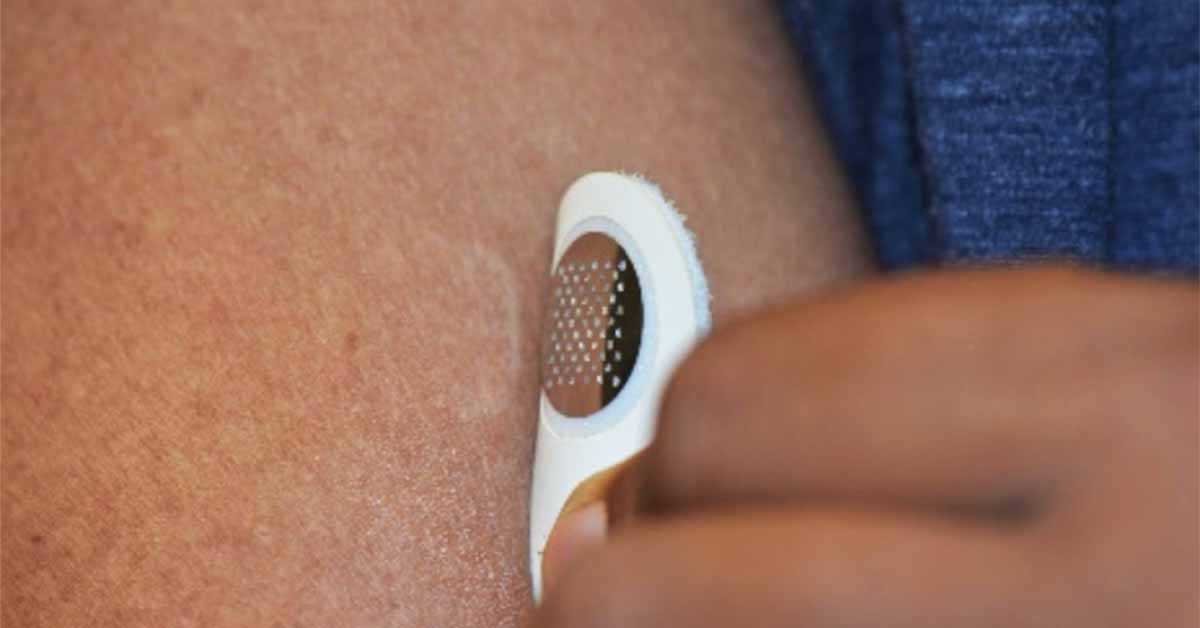
A Minimally Invasive Approach to Desensitization
MOON101 utilizes Moonlight’s proprietary intradermal allergen immunotherapy platform to desensitize individuals with a food allergy. This innovative platform is designed to modulate the patient’s immune system away from anaphylactic reactions that can be triggered by even small, accidental exposure to peanuts.
The therapy works by delivering allergens directly into the skin using a small, minimally invasive dermal stamp. This approach is designed for at-home self-administration, requiring less than five minutes of wear time, making it a potentially convenient and patient-friendly treatment option. The company’s platform has also received support to expand its broader pipeline development beyond MOON101.
Clinical Trial Set to Begin
The FDA clearance will enable the immediate start of the company’s first clinical trial, named SURVEYOR. The trial’s primary aim will be to evaluate the safety of MOON101 in peanut allergic adults and children and will be conducted in collaboration with leading food allergy centers in the United States.
Said Samir Patel, Co-founder and CEO of Moonlight Therapeutics:
This FDA clearance marks a pivotal milestone for Moonlight and for families living with peanut allergy. We are now positioned to move MOON101 into the clinic and evaluate its safety in peanut allergic individuals with the eventual goal of demonstrating that MOON101 can modulate the immune system.
Dr Brian Vickery, Chief of the Division of Allergy/Immunology at Children’s Healthcare of Atlanta and the lead clinical investigator on the trial, echoed the excitement for the new approach:
We are excited to begin testing the safety and tolerability of MOON101 in peanut-allergic individuals and evaluate its potential as a patient-friendly and convenient treatment option.
The SURVEYOR trial is supported in part by a cooperative grant from the National Institute of Allergy and Infectious Diseases.