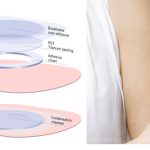
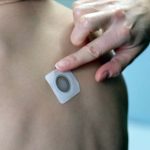
Home Search
viaskin peanut - search results
If you're not happy with the results, please do another search
Viaskin Peanut Phase III Trial Falls Short, Still Chance for FDA Approval
The Viaskin Peanut patch therapy still has 50:50 chance of approval according to research note.
Viaskin Peanut Patch: Lasting Response After Three Years
Pediatric patients responded with a favorable safety profile and no serious adverse effects.
Viaskin Peanut, Milk and Egg Patch Therapies: Promising News from the AAAAI Conference
We are cautiously optimistic that a safe, effective, easy to administer treatment for food allergy may be on the horizon.
Optimism Grows for Toddlers with Peanut Allergies as Viaskin Patch Safety Study Begins
DBV Technologies anticipates their submission to the FDA will occur in the second half of 2026
DBV Confirms Alignment with FDA on Accelerated Approval Pathway for the Viaskin® Peanut Patch in...
Toddlers study on-track to initiate in 2Q 2025.
DBV Announces Positive Regulatory Updates for the Viaskin® Peanut Patch in the United States and...
Company to pursue an Accelerated Approval pathway for toddlers ages 1–3 years-old.
DBV Announces Promising 2-Year Results from Ongoing Viaskin “Peanut Patch” Toddler Study
81.3% of subjects who completed the oral food challenge reached an eliciting dose of ≥1,000 mg after 24 months of treatment.
Will the Viaskin “Patch” Therapy for Peanut Allergy be Approved Soon? The Company is...
Company is restructuring in anticipation of delays.
Viaskin Patch Delivers Long-Lasting Peanut Desensitization After Phase IIb Trial
The study was designed to assess the long-term efficacy and safety of the treatment in subjects that had graduated from their earlier VIPES trial.
Children’s Response to Peanut Patch Therapy Increased Over 60 Months
VP250 may lead to an accumulation of clinical benefit with high treatment compliance, making this a valuable option for children with allergies.