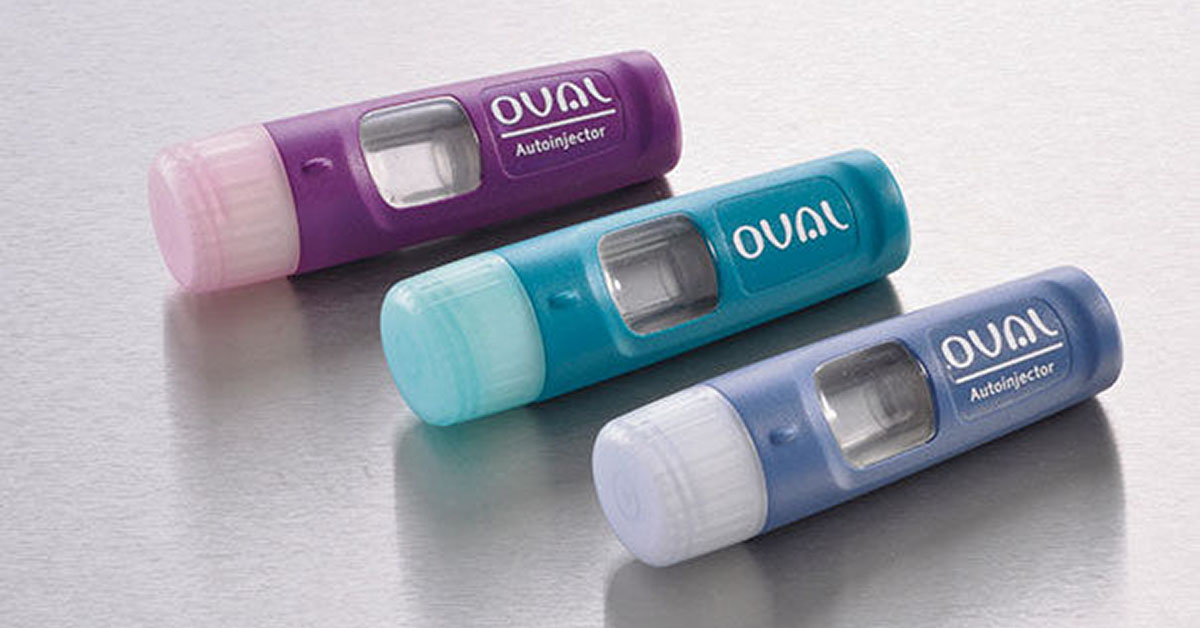
As recent events plainly indicate, consumers are painfully aware of the lack of competition and choice in the marketplace for epinephrine auto-injectors, the only drug indicated when life-threatening anaphylaxis strikes.
The problem was further exacerbated when Sanofi recalled their popular Auvi-Q device and announced an exit from the market in February. Since then, we have been reporting on alternatives making their way through the development and FDA approval process.
If you are interested in keeping abreast of the latest in auto-injector alternatives, keep your eye on our blog or subscribe to our Newsletter for periodic updates:
Here are articles we published in the past year on four noteworthy auto-injector designs currently at various stages in the pipeline with more to come:
/2016/07/epinephrine-auto-injectors-where-is-the-long-promised-generic/
/2016/06/delivering-the-next-generation-auto-injector/
/2015/12/patent-issued-for-innovative-auto-injector-mechanism/
/2016/01/medical-device-company-tests-compact-auto-injector-design/
/2016/07/adamis-auto-injector-rejected-by-fda/