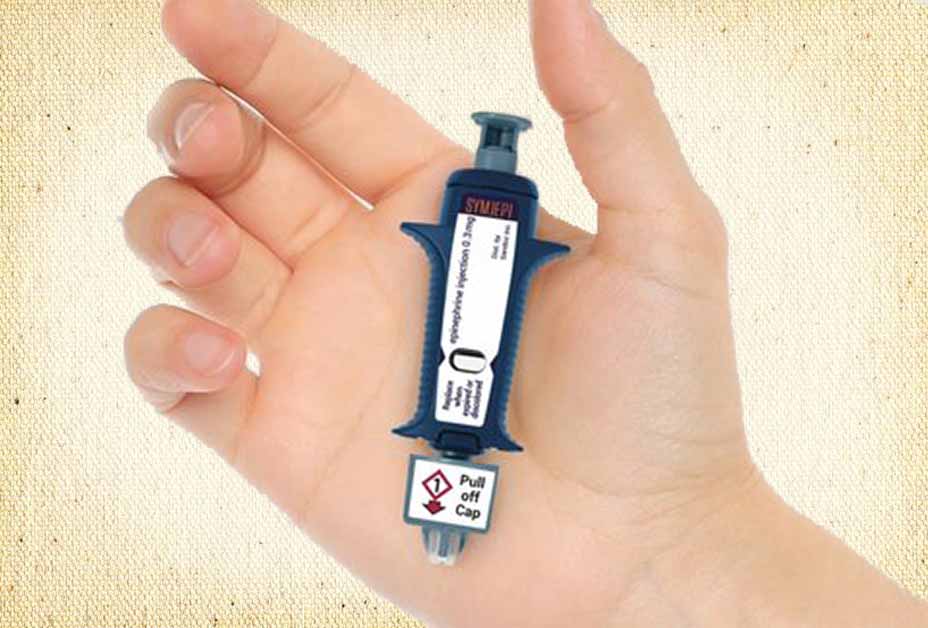
Note: The Symjepi emergency epinephrine device is not an auto-injector per se. It is a syringe prefilled with epinephrine with an exposed needle. Below, following the press release, is a video showing how Symjepi is administered in an emergency.
SYMJEPI Products Now Available in the Walgreens Prescription Savings Club, with the Lowest Prices for Epinephrine Products
SAN DIEGO, Jan. 21, 2021 (GLOBE NEWSWIRE) — Adamis Pharmaceuticals Corporation (NASDAQ: ADMP) today announced that its SYMJEPI® (epinephrine) Injection products are now available to members of the Walgreens Prescription Savings Club program, for a discounted price of $99.99 for a two-pack, the lowest price offered for epinephrine products through the Walgreens Prescriptions Savings Club, and the lowest price for epinephrine devices on the market1.
At the start of 2021, Adamis’ SYMJEPI 0.3mg and SYMJEPI 0.15mg Injection products, which are used in the emergency treatment of acute allergic reactions, were added to the Walgreens Prescription Savings Club program. The Walgreens Prescription Savings Club offers customers, who pay an annual membership fee, significant savings off retail prices on thousands of medications. For full details visit www.walgreens.com/psc/prescription-savings-club.
Dr. Dennis J. Carlo, President and Chief Executive Officer of Adamis Pharmaceuticals, added, “We are very excited Walgreens has added SYMJEPI through its Prescription Savings Club and feel this program will avail this potentially life-saving medication to the patients that need it most. Adamis remains committed to making SYMJEPI the most affordable epinephrine product available to patients.”
Important Safety Information
Contraindications
None.
Warnings and Precautions
You should get emergency medical care right away after using SYMJEPI.
You may need to use a second SYMJEPI (epinephrine) injection if symptoms continue or recur. Only a healthcare provider should give additional doses of epinephrine if you need more than 2 injections for a single anaphylaxis episode.
SYMJEPI should only be injected into the middle of your outer thigh (upper leg) with the needle facing downwards. Never inject into any other part of the body. If you accidentally inject SYMJEPI into any other part of your body, go to the nearest emergency room right away. Tell the healthcare provider where on your body you received the accidental injection. SYMJEPI can be injected through your clothing if needed.
The needle cap on the SYMJEPI prefilled syringe helps to prevent needle sticks and accidental injection of epinephrine. Do not remove the needle cap until you are ready to use it.
Never put your thumb, fingers, or hand over the exposed needle.
If an accidental injection happens, get medical help right away.
Do not drop the carrier case or SYMJEPI prefilled syringe. If the carrier case or prefilled syringe is dropped, check for damage and leakage. Dispose of the prefilled syringe and carrier case, and replace if damage or leakage is noticed or suspected.
Do not place patient information or any other foreign objects in the carrier case with the prefilled syringe, as this may prevent you from removing the prefilled syringe for use.
If you inject a young child with SYMJEPI, hold their leg firmly in place before and during the injection to prevent injuries. Ask your healthcare provider to show you how to properly hold the leg of a young child during injection.
Before using SYMJEPI, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems or high blood pressure.
- have diabetes.
- have thyroid problems.
- have asthma.
- have a history of depression.
- have Parkinson’s disease.
- are pregnant or plan to become pregnant. It is not known if epinephrine will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if epinephrine passes into your breast milk.
Adverse Reactions
SYMJEPI may cause serious side effects.
Rarely, patients who have used SYMJEPI may develop infections at the injection site within a few days of an injection. Some of these infections can be serious. Call your healthcare provider right away if you have any of the following at an injection site:
- redness that does not go away
- swelling
- tenderness
- the area feels warm to the touch
Cuts on the skin, bent needles, and needles that remain in the skin after the injection, have happened in young children who do not cooperate and kick or move during the injection. If you have certain medical conditions, or take certain medicines, your condition may get worse or you may have longer lasting side effects when you use your SYMJEPI. Talk to your healthcare provider about all your medical conditions.
Common side effects of SYMJEPI include:
- fast, irregular or “pounding” heartbeat
- sweating
- headache
- weakness
- shakiness
- paleness
- feelings of over excitement, nervousness or anxiety
- dizziness
- nausea and vomiting
- breathing problems
These side effects may go away with rest. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of SYMJEPI. For more information, ask your healthcare provider or pharmacist.
Drug Interactions
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Tell your healthcare provider of all known allergies.
Especially tell your healthcare provider if you take certain asthma medicines.
SYMJEPI and other medicines may affect each other, causing side effects. SYMJEPI may affect the way other medicines work, and other medicines may affect how SYMJEPI works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
Please see full Prescribing Information for SYMJEPI.
To report SUSPECTED ADVERSE REACTIONS, contact Adamis Pharmaceuticals Corporation at 1-800-230-3935 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
About Adamis Pharmaceuticals
Adamis Pharmaceuticals Corporation is a specialty biopharmaceutical company primarily focused on developing and commercializing products in various therapeutic areas, including allergy, opioid overdose, respiratory and inflammatory disease. The company’s SYMJEPI (epinephrine) Injection products are approved by the FDA for use in the emergency treatment of acute allergic reactions, including anaphylaxis. In addition to its ZIMHI, naloxone injection product candidate, Adamis is developing additional products, including treatments for acute respiratory diseases, such as COVID-19, influenza, asthma and COPD. The company’s subsidiary, U.S. Compounding, Inc., compounds sterile prescription drugs, and certain nonsterile drugs for human and veterinary use by hospitals, clinics, surgery centers, and vet clinics throughout most of the United States.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include those that express plans, anticipation, intent, contingencies, goals, targets or future development and/or otherwise are not statements of historical fact. These statements relate to future events or future results of operations, including, but not limited to the following statements: the company’s beliefs concerning the commercialization of SYMJEPI and its other products and product candidates; the extent of sales of SYMJEPI resulting from the availability of the products within the Walgreens Prescription Savings Club program; and the company’s beliefs concerning the ability of its product to compete successfully in the market; the company’s beliefs concerning the safety and effectiveness of SYMJEPI or its other products and product candidates. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, which may cause Adamis’ actual results to be materially different from these forward-looking statements. You should not place undue reliance on any forward-looking statements. There are no assurances regarding the extent of sales of SYMJEPI products, or revenues to the company, resulting from the availability of the products within the Walgreens Prescription Savings Club program. Further, any forward-looking statement speaks only as of the date on which it is made, and except as may be required by applicable law, we undertake no obligation to update or release publicly the results of any revisions to these forward-looking statements or to reflect events or circumstances arising after the date of this press release. Certain of these risks, uncertainties, and other factors are described in greater detail in Adamis’ filings from time to time with the SEC, which Adamis strongly urges you to read and consider, all of which are available free of charge on the SEC’s web site at http://www.sec.gov. Except to the extent required by law, any forward-looking statements in this press release speak only as the date of this press release, and Adamis expressly disclaims any obligation to update any forward-looking statements.
1ProspectoRx data for epinephrine devices, December 2020.
Contacts:
Mark Flather
Senior Director, Investor Relations
& Corporate Communications
Adamis Pharmaceuticals Corporation
(858) 412-7951
mflather@adamispharma.com
Note of disclosure: Kaleo is an advertiser with SnackSafely.com