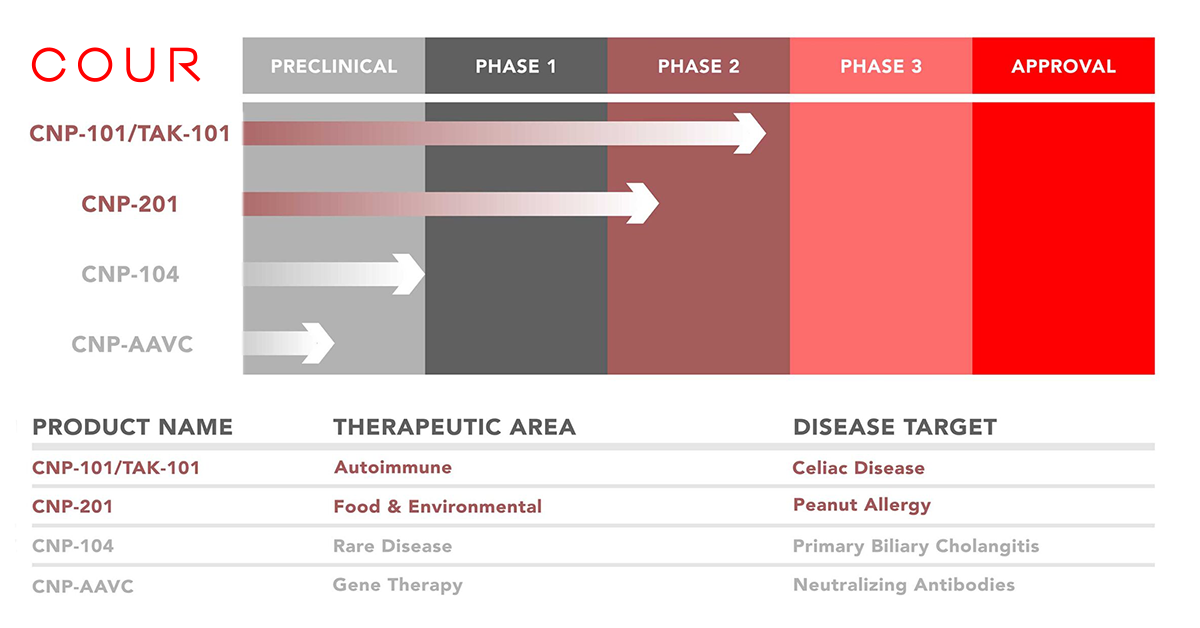
CNP-201 is a nanoparticle containing peanut protein that aims to eliminate allergic responses
CHICAGO, Oct. 19, 2021 /PRNewswire/ — COUR Pharmaceuticals, a biotechnology company developing novel immune-modifying nanoparticles to treat immune disorders (CNPs), today announced that the first patient has been dosed in the Company’s first-in-human proof-of-concept (Phase 1b/2a) of its lead candidate, CNP-201, for the treatment of peanut allergy. The trial will evaluate the safety and efficacy of CNP-201 in people ages 16 to 55 with peanut allergy and is led by Kari Nadeau, M.D., Ph.D., Director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University.
“Dosing of our first patients with CNP-201 is an important milestone for COUR Pharmaceuticals, as it brings us one step closer to finding a cure for the most common food allergy in children under age 18 and the second-most common food allergy in adults,” said John J. Puisis, Co-Founder, President & CEO of COUR. “This study will produce important clinical data to guide COUR’s future and continues to demonstrate our commitment to our patients.”
A recent study in the Journal of Allergy and Clinical Immunology found that as many as 4.6 million U.S. adults are allergic to peanuts. The immune systems of people with peanut allergy can mount an abnormal immune response to even tiny amounts of peanuts and trigger a serious reaction in minutes leading to life-threatening anaphylaxis.
“Medical Research of Arizona, the research division of Allergy, Asthma & Immunology Associates, is excited to have enrolled and dosed the first patient in the CNP-201-5.001 peanut allergy clinical study. This study will bring new therapeutic options to our patients suffering from peanut allergies,” said Dr. Michael Manning, a Principal Investigator and CEO at Medical Research of Arizona. “Peanut allergy is a Th2 cell mediated immune response against peanut protein epitopes. The potential for CNP-201 to inhibit the peanut specific Th2 cell response through immune reprogramming could potentially abrogate peanut allergy and thereby reduce the burdens associated with a lifetime peanut-free diet and the serious risk of fatality associated with severe allergic reaction to peanuts. We look forward to our continued contributions to this groundbreaking study, which could possibly displace OIT Therapy.”
CNP-201 is a biodegradable nanoparticle encapsulating purified peanut protein extract and administered through intravenous infusion. The nanoparticles containing peanut allergens are consumed by immune presenting cells. When the particles and allergens are presented within the immune processing cell, they lead to a reprogramming of the cellular functions of the immune system, reducing and possibly eliminating the potential risk of severe allergic reactions.
COUR’s breakthrough nanoparticle immune modifying platform, which preserves all immune functionality, is potentially applicable in treating any autoimmune or allergic condition.
Active and Enrolling Clinical Sites:
- Allergy and Asthma Associate of Santa Clara Valley- Alan Goldsobel (http://www.allergycare.com)
- Allergy and Asthma, San Diego- Alexander Griener, MD (https://allergyandasthma.com)
- Dallas Allergy and Asthma- Gary Gross, MD (https://www.daac-prc.com)
- Medical Research of Arizona, Scottsdale- Michael Manning, MD (https://www.medicalresearchaz.com)
- Peninsula Research Associates, Los Angeles-Lawrence Sher, MD (http://peninsularesearch.com)
- Portland Allergy and Asthma Clinic- Stephen B. Fritz, MD (https://www.portland-allergy.com)
- Seattle Allergy and Asthma- Dan Petroni, MD (https://seattleallergy.org/)
- Stanford University- Sharon Chinthrajah, MD
- Virginia Mason Medical Center- David Jeong, MD
- Western Sky Medical Research, El Paso- Todd Funkhouser, MD (https://www.westernskymedicalresearch.com)
About COUR Pharmaceuticals
COUR Pharmaceuticals is developing first-in-class therapies designed to reprogram the immune system to achieve antigen-specific tolerance for immune-mediated disease. COUR’s platform of immune-modifying nanoparticles treats the root cause of immune disease, unlike traditional approaches, which only minimize symptoms using toxic immune suppression. COUR’s lead product for celiac disease, partnered with Takeda Pharmaceutical Company, is the first demonstration of induction of antigen-specific immune tolerance in any autoimmune disease. Data from clinical and preclinical settings demonstrate the opportunity for the COUR nanoparticle platform to address a wide range of immune and inflammatory conditions. The underlying technology was acquired from Northwestern University and draws from more than 30 years of research by the laboratory of Stephen D. Miller, Ph.D., the Judy E. Guggenheim Research Professor of Microbiology-Immunology.
Media Contact:
Julie Ferguson
Julie@jfprmedia.com
(312) 385-0098