Sandoz, a US-based division of Novartis AG, has announced the launch date for Symjepi, their emergency epinephrine device. The 0.3mg “adult dose” version will be available in the first quarter of 2019 with lower “child” and “pediatric” doses to follow.
Sandoz has set the wholesale price of the device at $250, a 16% discount off the $300 price of Mylan’s EpiPen generic and the recently announced Teva generic which has begun selling in limited quantities in the US.
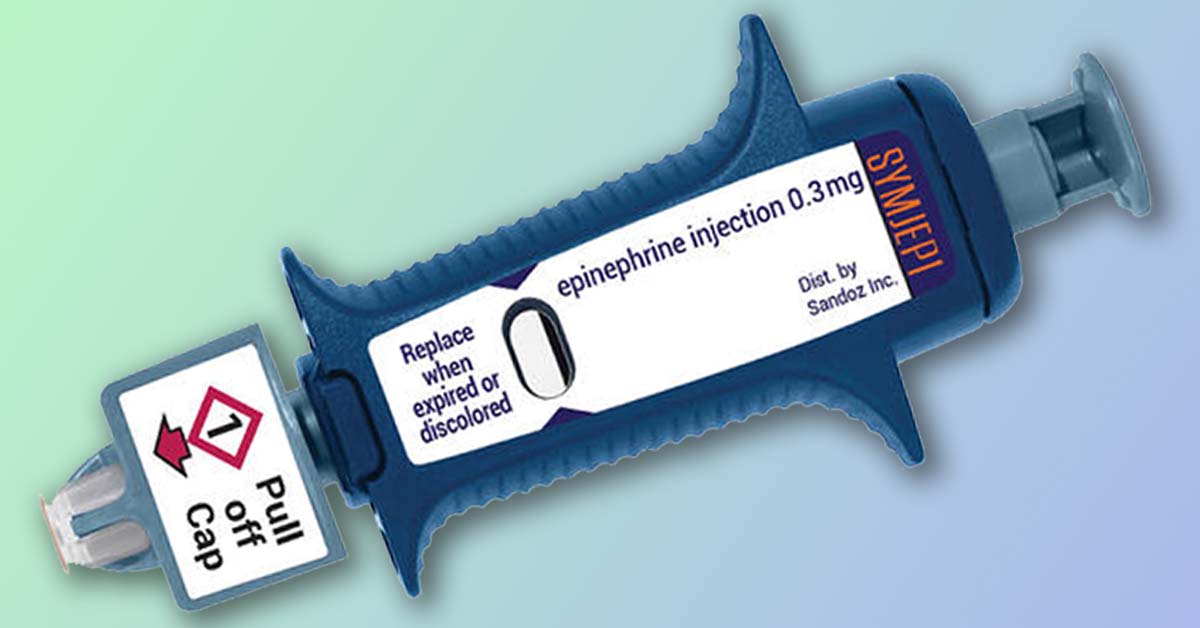
Despite news reports, it should be noted that Symjepi is not an auto-injector per se but a prefilled syringe. As such, it does not have a retractable needle and does not administer epinephrine automatically. Instead, one must pull the cap, insert the needle, press the plunger until the drug has been administered, then remove the needle, much like one does with traditional syringes. Because the device is a prefilled syringe, it is better suited to administration by a trained adult such as a nurse or first responder.
With the knowledge that Symjepi is a much simpler device requiring more coordination to administer, the expectation was that it would be priced significantly lower than the auto-injectors it is set to compete with, driving the cost of emergency epinephrine downward. The announced $250 price tag dispels that notion.
The debut of Symjepi is a welcome addition of another emergency epinephrine device from a manufacturer other than Pfizer, the company that has experienced manufacturing problems leading to shortages of EpiPen and the Adrenaclick generic this summer. Symjepi joins Kaleo’s Auvi-Q and Teva’s generic as Pfizer alternatives.
The FDA approved Symjepi back in June of 2017, but Adamis Pharmaceuticals – the manufacturer of the device – had difficulties engaging a partner to commercialize the device and bring it to market. The company finally announced their partnership with Sandoz this July.