DBV Technologies, the firm that received the Food and Drug Administration’s (FDA) Breakthrough Therapy Designation for their peanut patch in April, issued a press release stating that the board overseeing their milk patch Phase I trial found no safety concerns and is recommending the therapy progress on to Phase II.
Phase I studies focus on the safety of a new therapy while Phase II studies focus on a therapy’s efficacy. Pending a review of the Phase I data by the FDA and approval of the proposed Phase II protocol, the firm expects to continue on to the Phase II study in the second half of 2015.
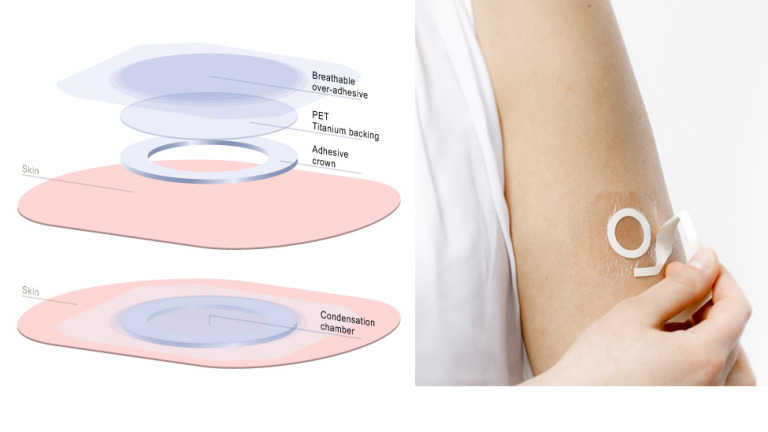
DBV describes Viaskin® as “an electrostatic patch, based on Epicutaneous Immunotherapy, or EPIT®, which administers an allergen directly onto the superficial layers of the skin to activate the immune system by specifically targeting antigen-presenting cells without allowing passage of the antigen into the bloodstream.” In other words, the patch therapy introduces increasing quantities of an allergen through the skin and by doing so desensitizes the individual to that allergen.
While this is encouraging news for those suffering with milk allergy, please keep in mind that even if the therapy is found to be effective, it will be several years before the therapy becomes available.