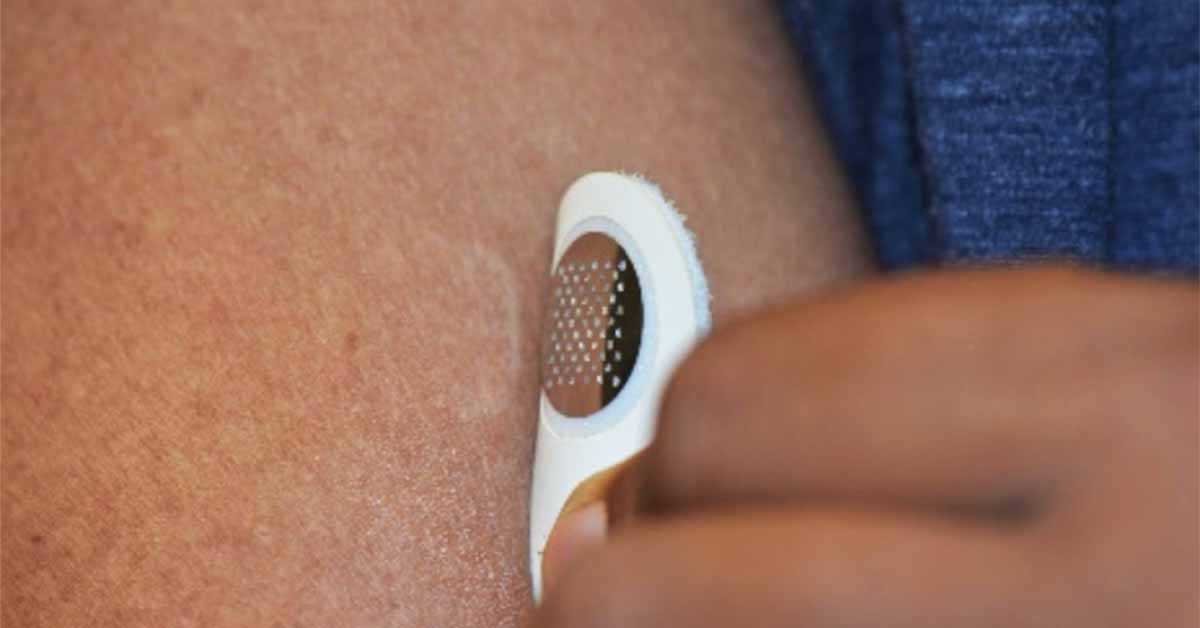
A five-minute weekly dose of peanut protein through the skin, delivered by a novel microneedle technology, was superior in desensitizing peanut-allergic mice compared to administering the protein through a skin patch, a new study has found.
The results of the study at the University of Michigan’s Mary H. Weiser Food Allergy Center (MHWFAC) were published February 24 in the journal Immunotherapy.
Investigators believe that microneedle devices may lead to improved allergen immunotherapy for human patients.
“This is a very interesting technology that could provide a safer method to desensitize people with food allergies,” said James R. Baker, Jr., M.D., an immunologist and director of the MHWFAC. “These successful animal results argue for further development of this platform.”
Peanut allergy is a potentially deadly condition with few treatment options. In recent years, significant progress has been made in the development of allergen-specific immunotherapies, which desensitize the patient with peanut allergy through repeated exposure to peanut protein.
Currently, orally administered immunotherapy is the only treatment for peanut allergy approved by the U.S. Food and Drug Administration (FDA). However, it requires that patients follow a strict protocol for ingesting each dose.
Epicutaneous immunotherapy (EPIT) via patches worn on the skin for as much as 24 hours per day has been shown in clinical trials to be safe but provides limited efficacy – perhaps because of the tight barriers of the skin surface, which may limit the amount of allergen taken up by the body.
In the new study, investigators tested a dermal stamp, which is applied for a few minutes a day and uses microneedle (MN) technology to deliver peanut protein to immune cells within the skin. Previous studies in humans have shown the microneedle technology to be minimally invasive.
During the study, peanut-allergic mice received five weekly treatments of either the five-minute MN immunotherapy or a 24-hour EPIT treatment.
Mice that received five weekly treatments with the EPIT patch were not significantly protected from allergic reaction, while treatment duration of two months was required for efficacy with EPIT.
But mice that were treated five minutes per day with the peanut-coated microneedle device had significantly enhanced desensitization, providing protection from reactivity following peanut challenge. The results were achieved despite the microneedle therapy applying a dose of peanut protein ten times lower than the dose delivered by EPIT.
“While our pre-clinical results are from studies in animal models, they demonstrate the potential for peanut-MNs to improve food allergen immunotherapy through the skin,” said Jessica O’Konek, Ph.D., the study’s senior author and a research assistant professor at the MHWFAC and the Michigan Nanotechnology Institute for Medicine and Biological Sciences. “Treatment options for food allergy are limited, so there is a lot of motivation for the development of novel therapeutics. It will be exciting to watch the clinical development of this technology,” she said.
The microneedle device used in the study was based on the proprietary treatment platform TASIS (Targeted Allergen-Specific Immunotherapy within the Skin) developed by Atlanta-based Moonlight Therapeutics.
The biotechnology firm, which focuses on treatments for food allergies, in 2021 received a received a $1.9 million grant from the National Institute of Allergy and Infectious Diseases (NIAID) to pave the way for clinical studies.
Moonlight Therapeutics completed a pre-Investigational New Drug (IND) meeting with the U.S. Food and Drug Administration, and intends to proceed with clinical trials soon.
“We are encouraged by this data and our recent meeting with the FDA,” said Samir Patel, Ph.D., chief executive officer of Moonlight Therapeutics. “Our first trial will evaluate the safety and tolerability of this approach in adults and children with peanut allergy.”